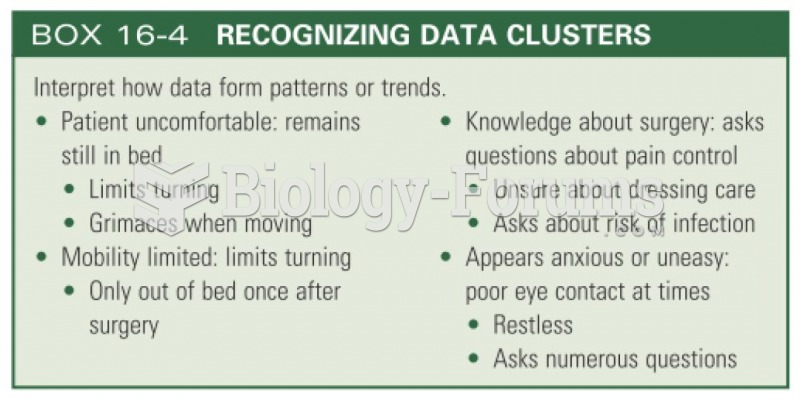
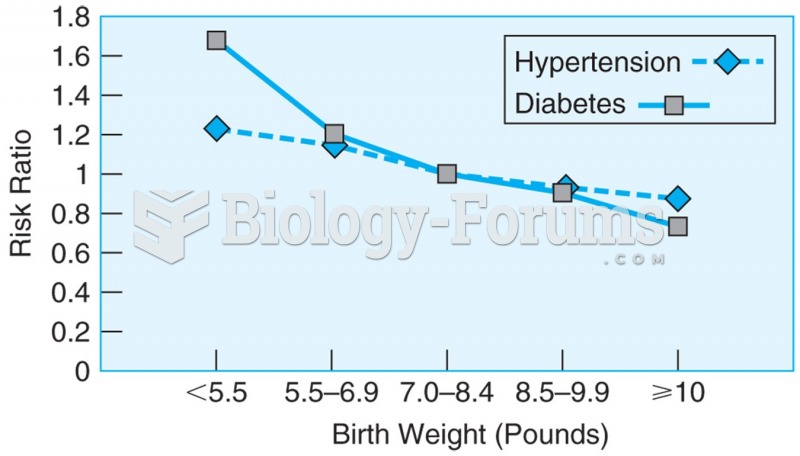
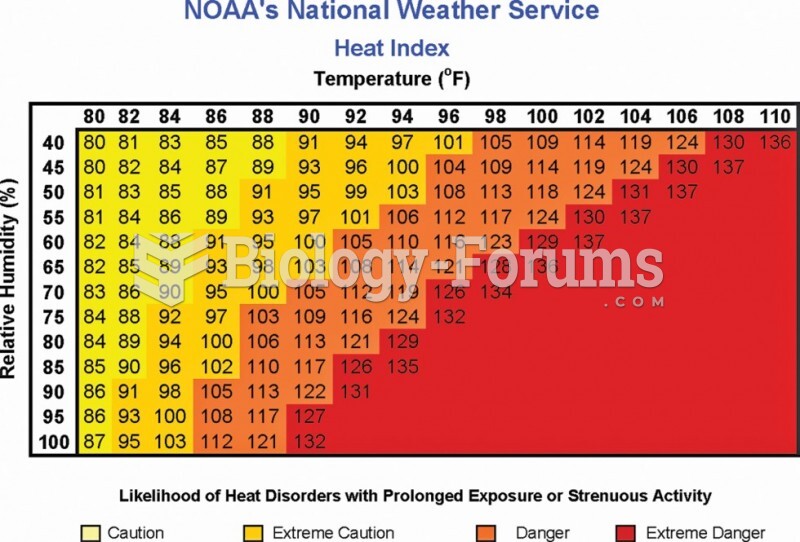
|
|
|
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.
On average, the stomach produces 2 L of hydrochloric acid per day.
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
Chronic necrotizing aspergillosis has a slowly progressive process that, unlike invasive aspergillosis, does not spread to other organ systems or the blood vessels. It most often affects middle-aged and elderly individuals, spreading to surrounding tissue in the lungs. The disease often does not respond to conventionally successful treatments, and requires individualized therapies in order to keep it from becoming life-threatening.
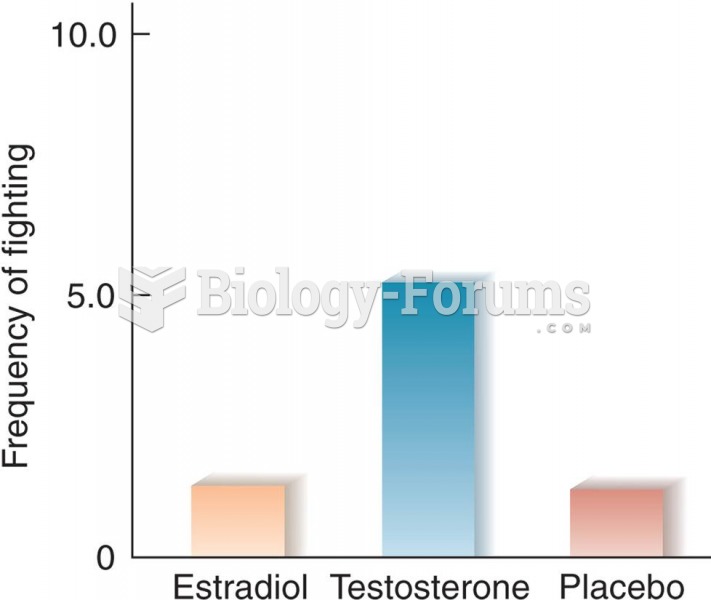 Effects of Estradiol and Testosterone on Interfemale Aggression in Rats (Based on data from van de P
Effects of Estradiol and Testosterone on Interfemale Aggression in Rats (Based on data from van de P
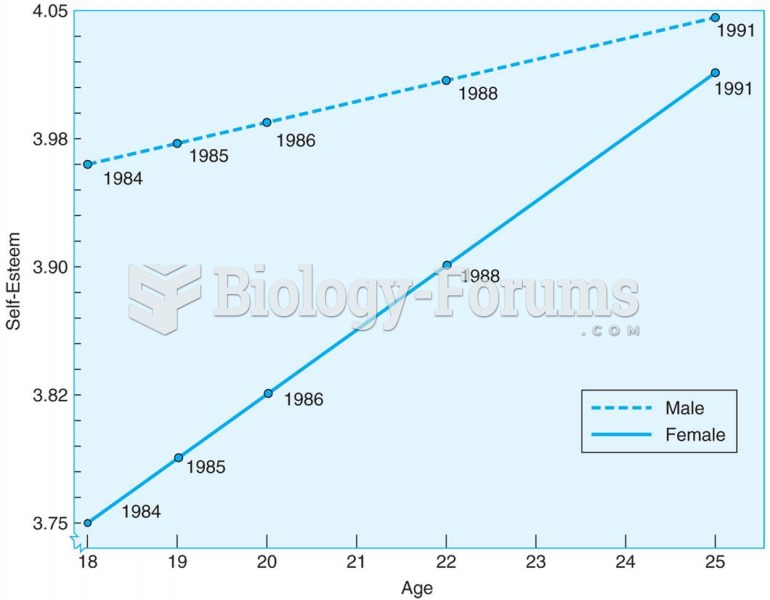 Young adults increase in self-esteem between the ages of 18 and 25, according to this longitudinal s
Young adults increase in self-esteem between the ages of 18 and 25, according to this longitudinal s