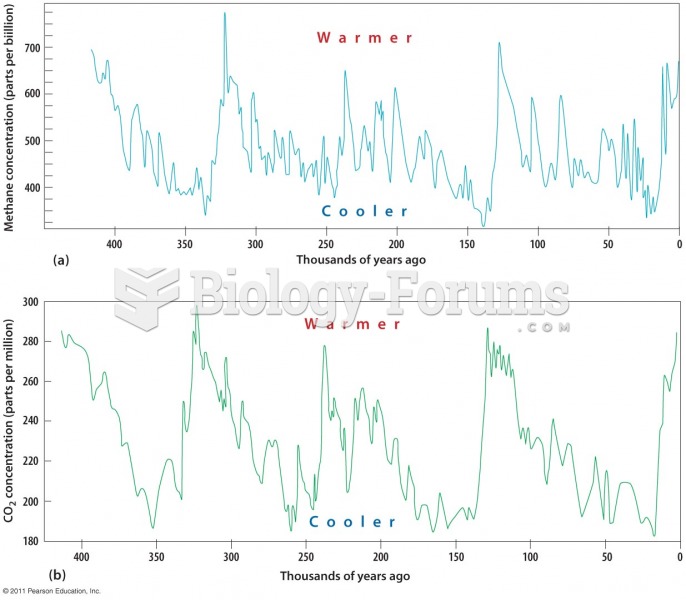
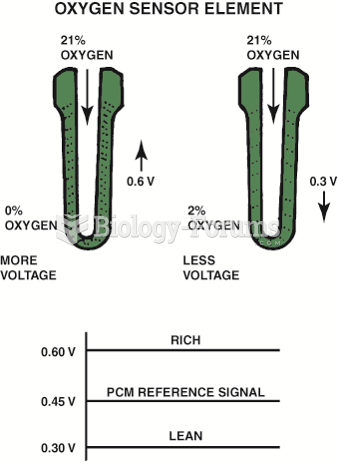
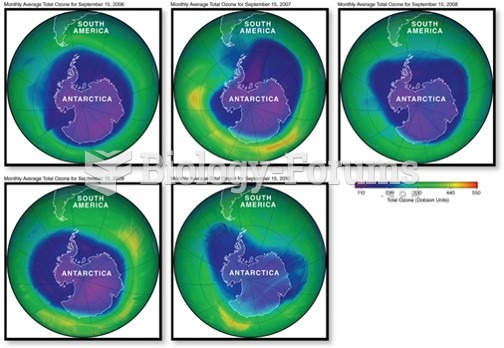
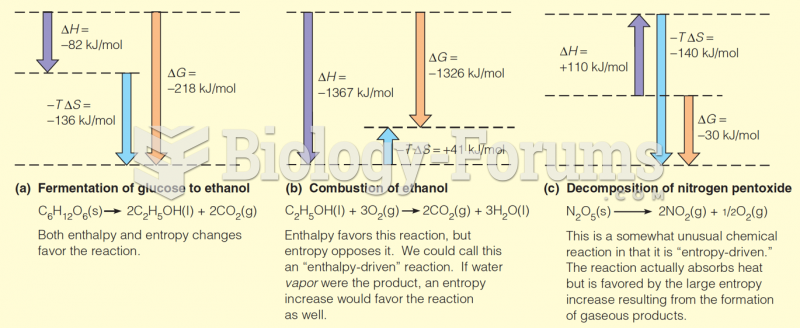
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Did you know?
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.