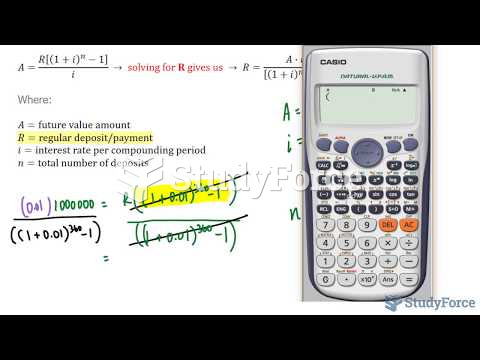
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.