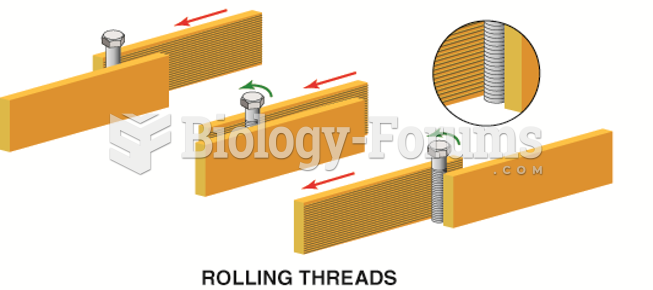
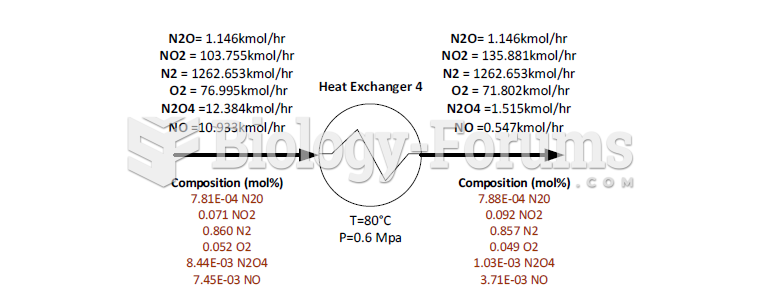
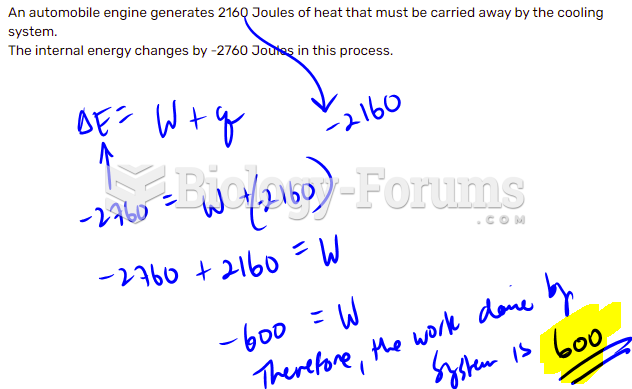
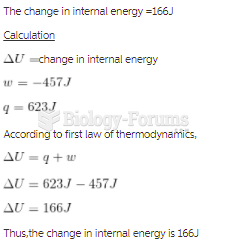|
|
|
In 2010, opiate painkllers, such as morphine, OxyContin®, and Vicodin®, were tied to almost 60% of drug overdose deaths.
Many people have small pouches in their colons that bulge outward through weak spots. Each pouch is called a diverticulum. About 10% of Americans older than age 40 years have diverticulosis, which, when the pouches become infected or inflamed, is called diverticulitis. The main cause of diverticular disease is a low-fiber diet.
Anesthesia awareness is a potentially disturbing adverse effect wherein patients who have been paralyzed with muscle relaxants may awaken. They may be aware of their surroundings but unable to communicate or move. Neurologic monitoring equipment that helps to more closely check the patient's anesthesia stages is now available to avoid the occurrence of anesthesia awareness.
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.