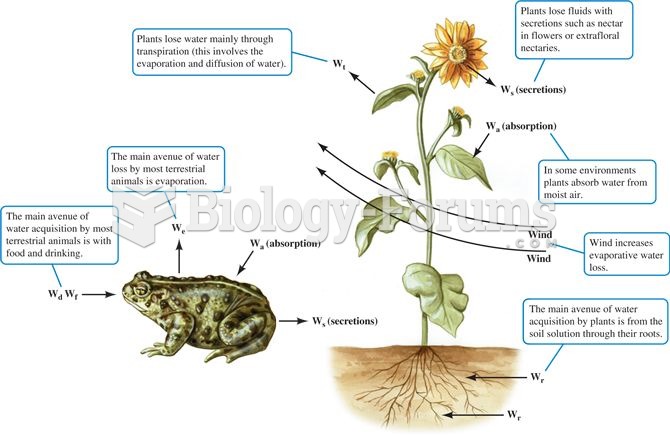
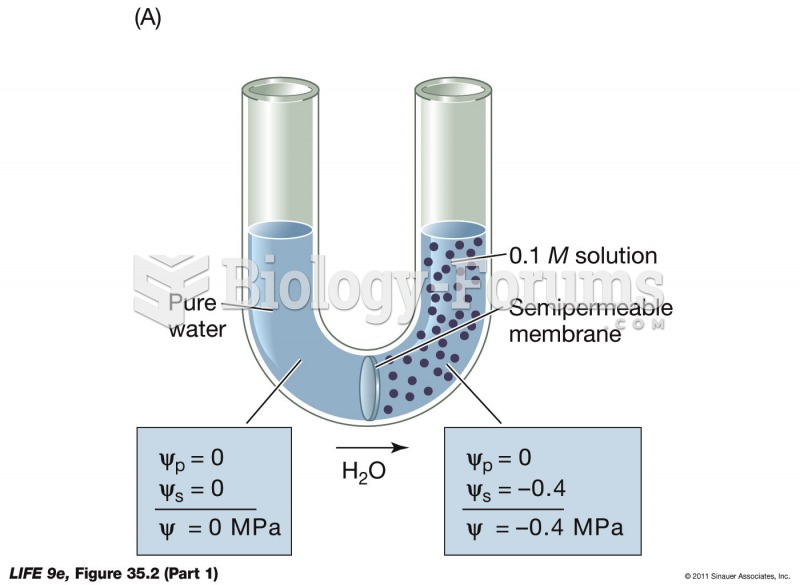
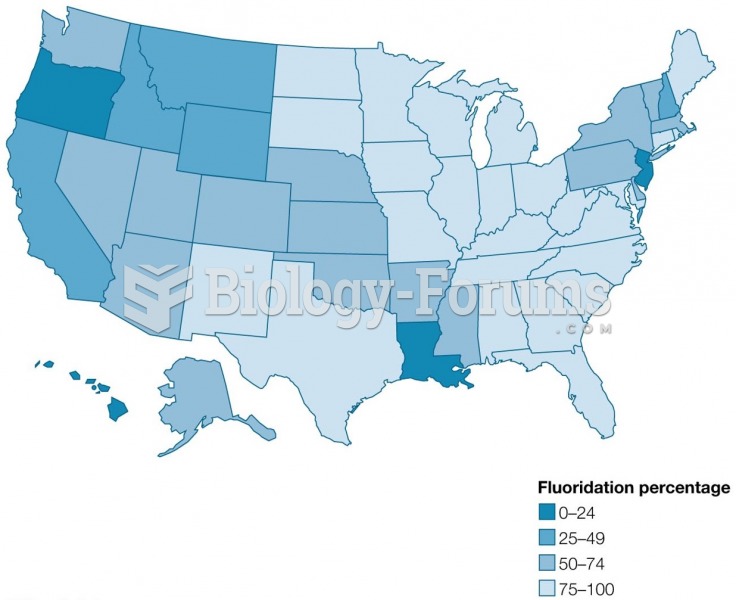
|
|
|
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.