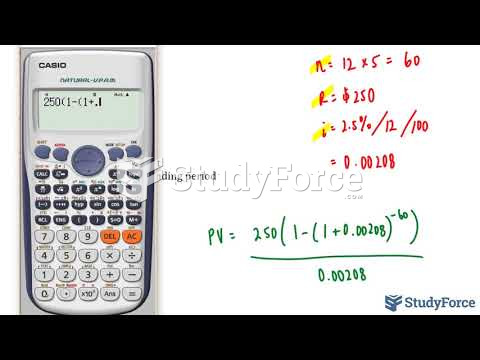
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.