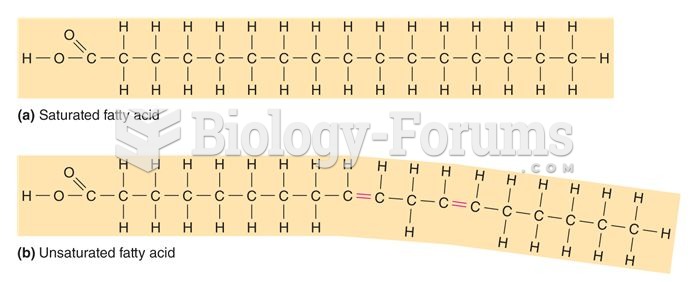
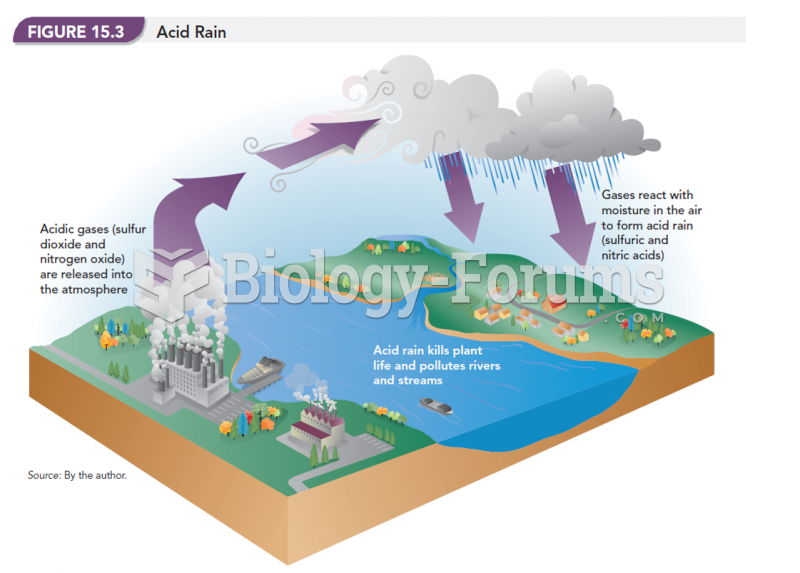
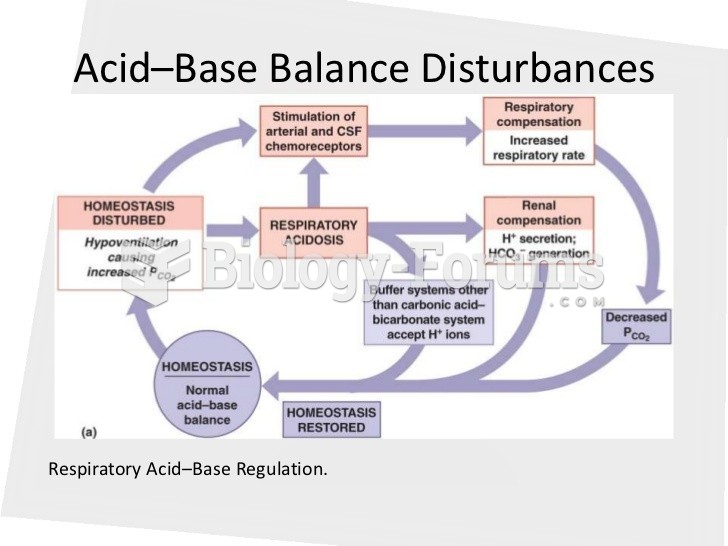
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.