This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
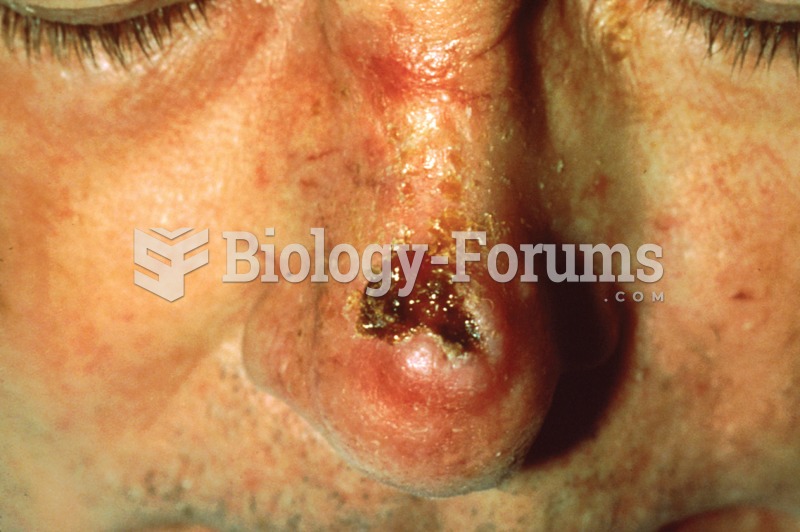
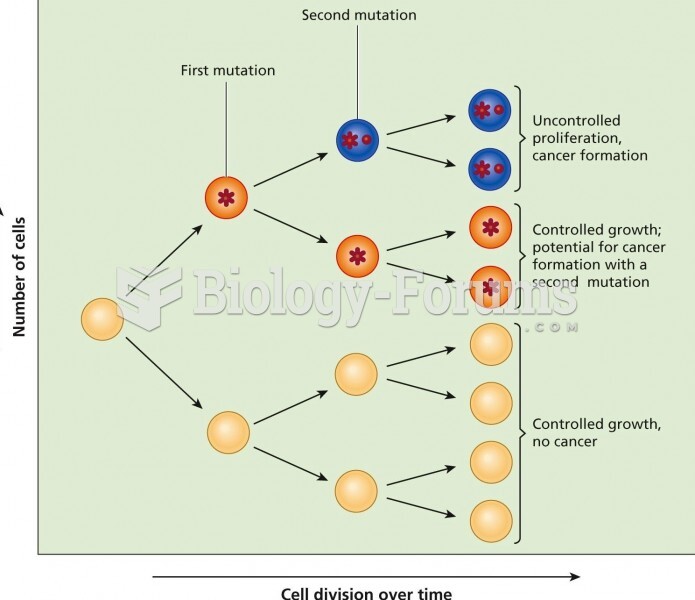
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
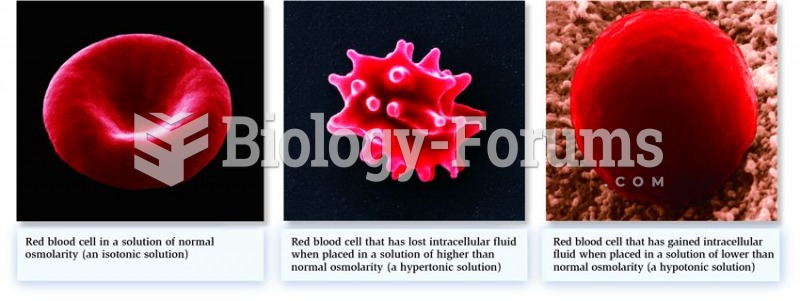
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
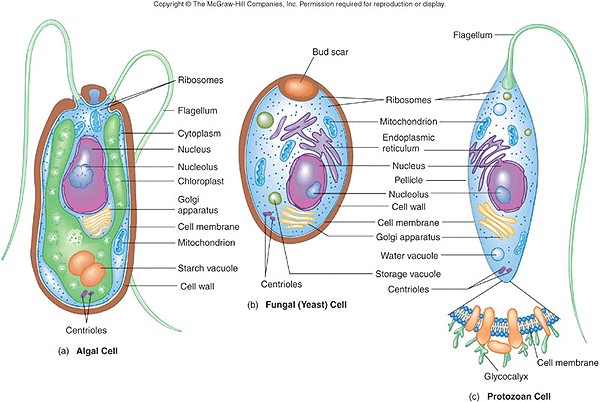
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
Did you know?
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.