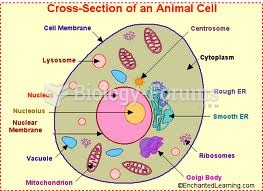
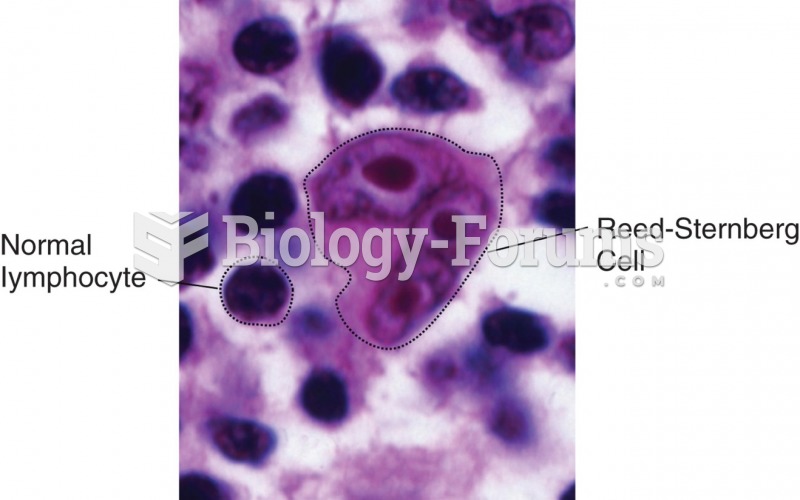
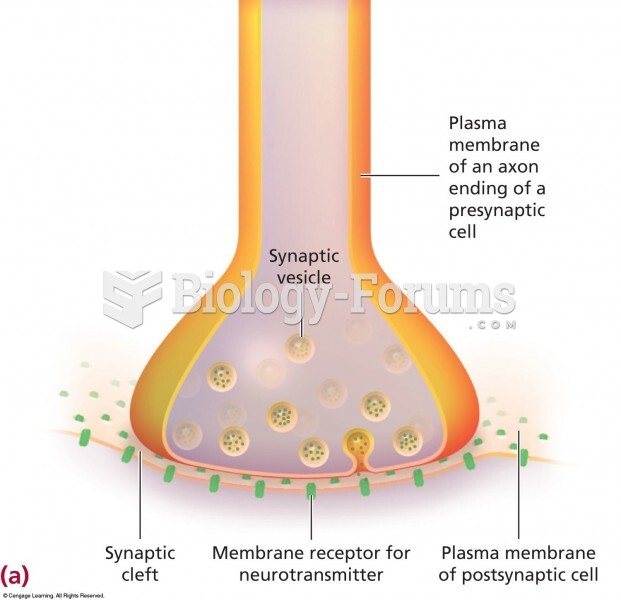
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
All adults should have their cholesterol levels checked once every 5 years. During 2009–2010, 69.4% of Americans age 20 and older reported having their cholesterol checked within the last five years.