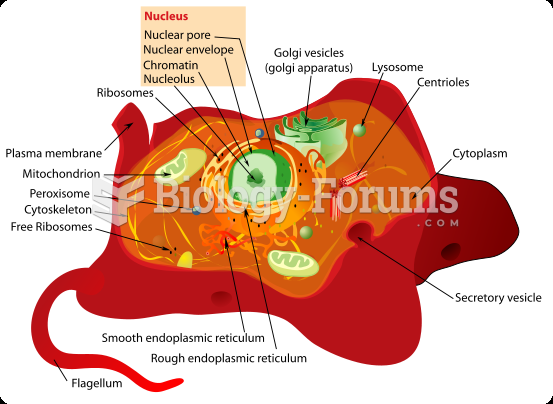
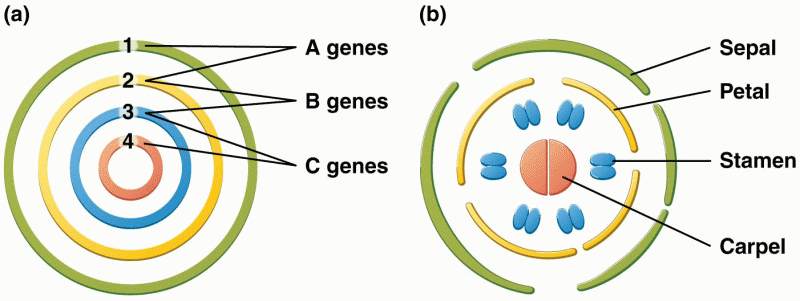
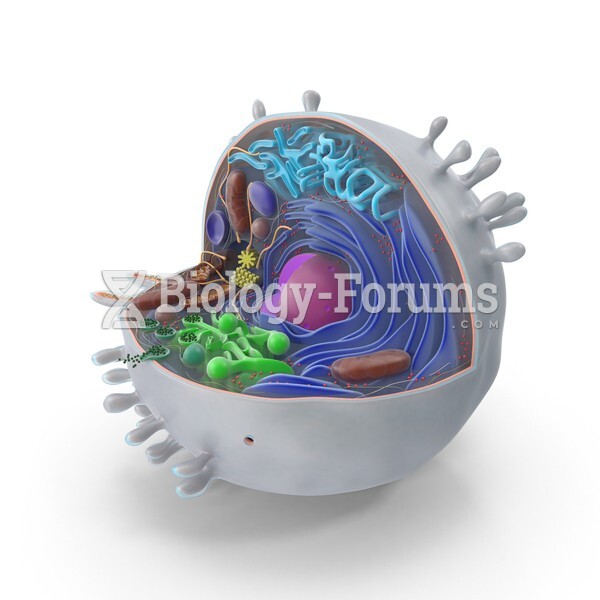
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Acute bronchitis is an inflammation of the breathing tubes (bronchi), which causes increased mucus production and other changes. It is usually caused by bacteria or viruses, can be serious in people who have pulmonary or cardiac diseases, and can lead to pneumonia.
Did you know?
Medication errors are three times higher among children and infants than with adults.