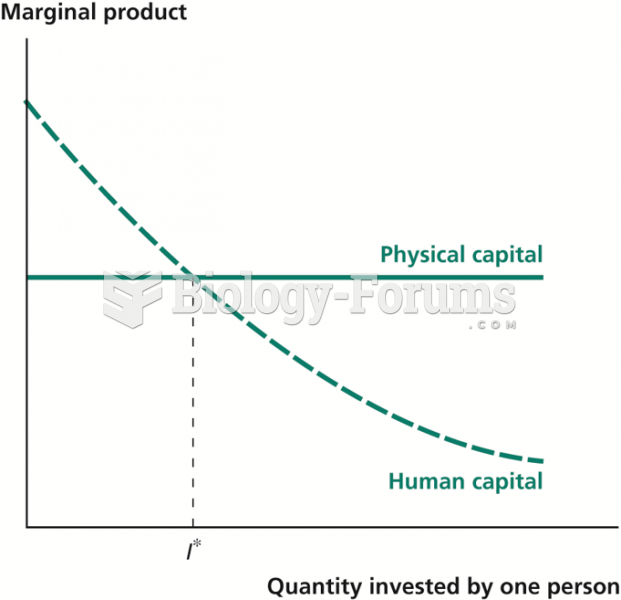
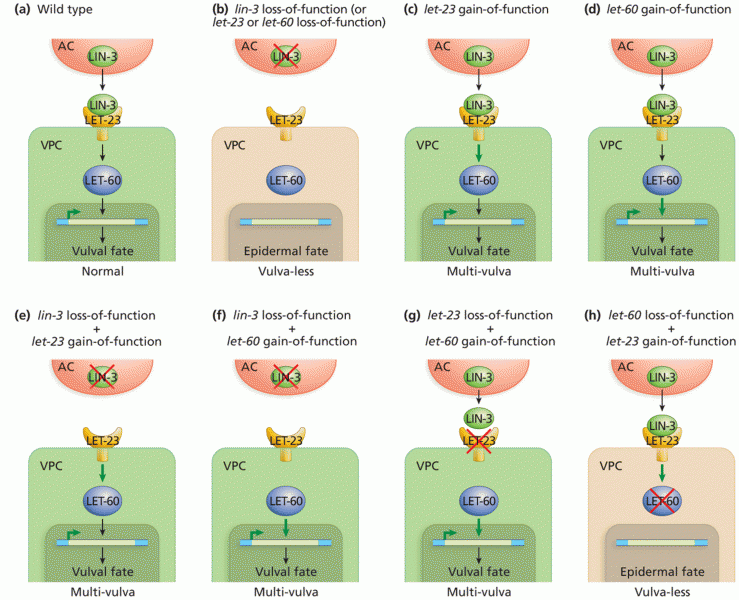
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.