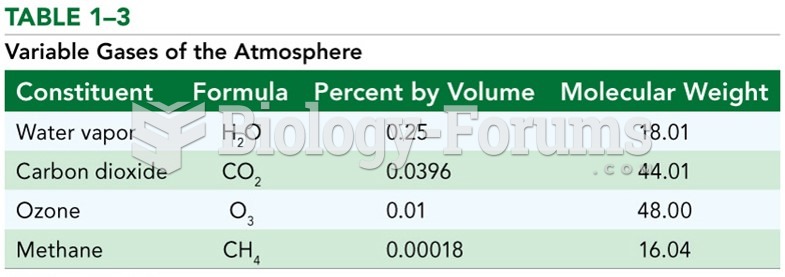
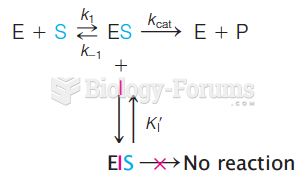
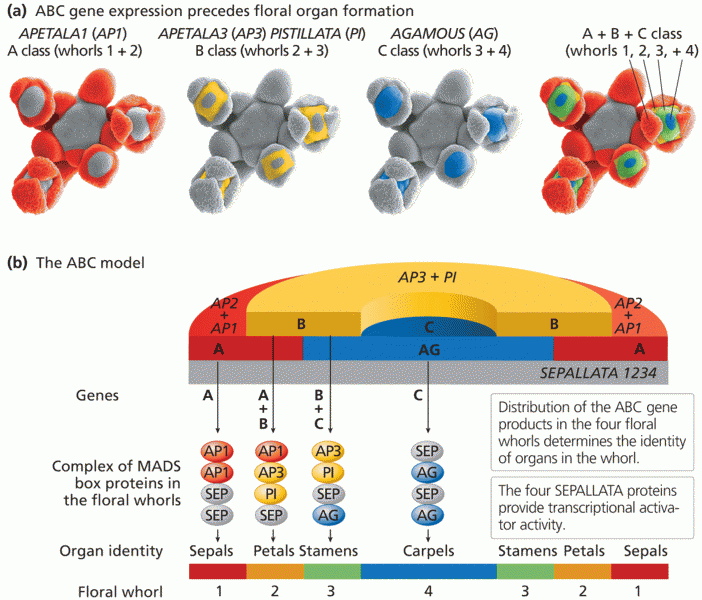
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
About 60% of newborn infants in the United States are jaundiced; that is, they look yellow. Kernicterus is a form of brain damage caused by excessive jaundice. When babies begin to be affected by excessive jaundice and begin to have brain damage, they become excessively lethargic.
Did you know?
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.