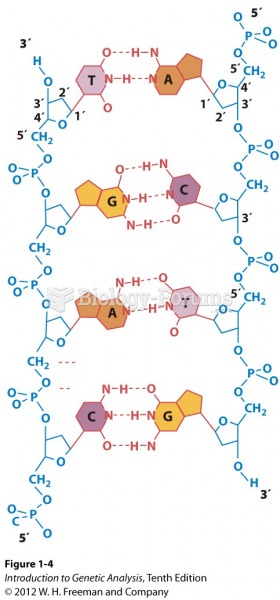
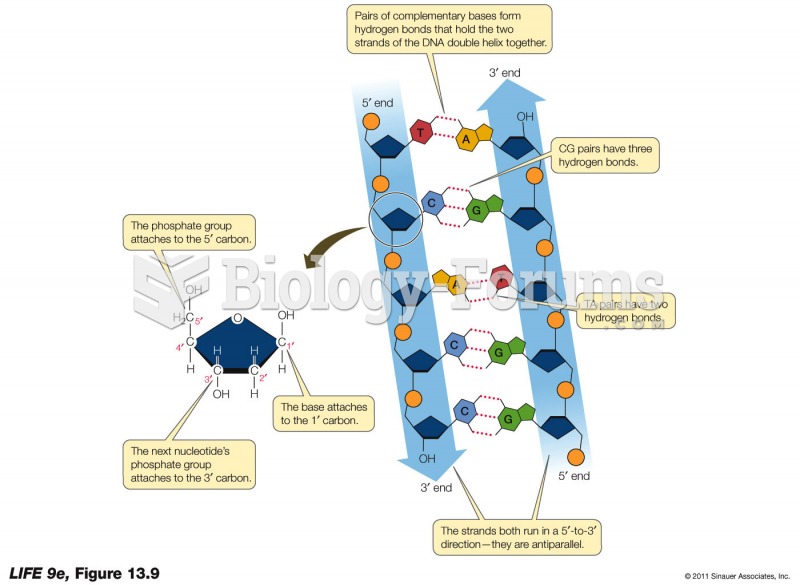
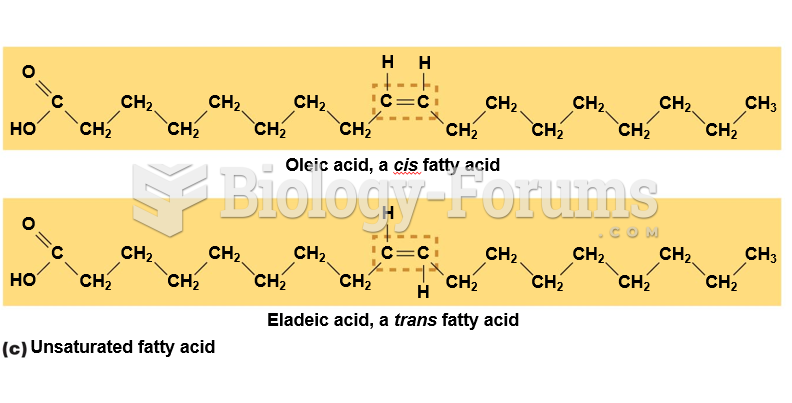
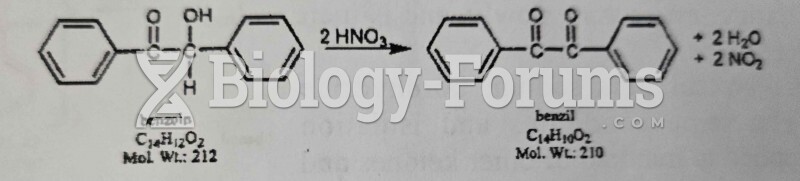
|
|
|
Cutaneous mucormycosis is a rare fungal infection that has been fatal in at least 29% of cases, and in as many as 83% of cases, depending on the patient's health prior to infection. It has occurred often after natural disasters such as tornados, and early treatment is essential.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Children of people with alcoholism are more inclined to drink alcohol or use hard drugs. In fact, they are 400 times more likely to use hard drugs than those who do not have a family history of alcohol addiction.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.