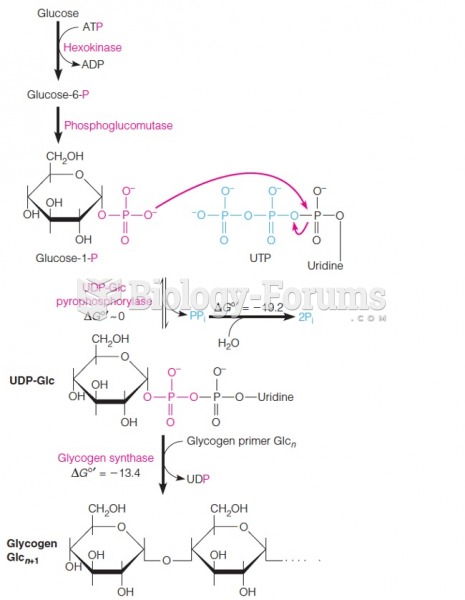
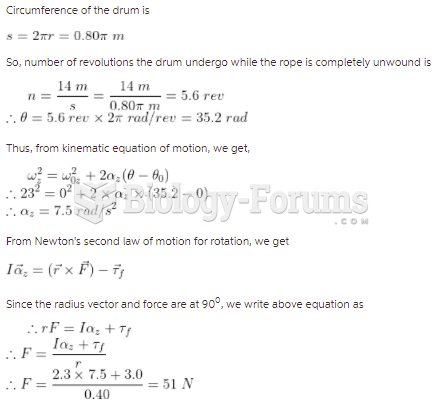
|
|
|
Though the United States has largely rejected the metric system, it is used for currency, as in 100 pennies = 1 dollar. Previously, the British currency system was used, with measurements such as 12 pence to the shilling, and 20 shillings to the pound.
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
Most strokes are caused when blood clots move to a blood vessel in the brain and block blood flow to that area. Thrombolytic therapy can be used to dissolve the clot quickly. If given within 3 hours of the first stroke symptoms, this therapy can help limit stroke damage and disability.
Approximately one in three babies in the United States is now delivered by cesarean section. The number of cesarean sections in the United States has risen 46% since 1996.
 These two species of bedstraw grow predominately on different soil types: Galium saxatile (shown her
These two species of bedstraw grow predominately on different soil types: Galium saxatile (shown her
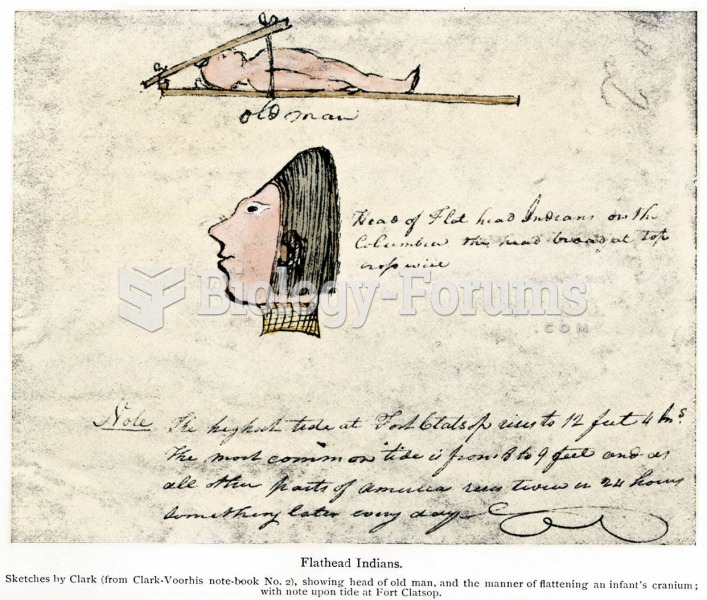 The “Flat Head” (Chinook) Indians acquired their name through shaping in infancy, as shown in a diag
The “Flat Head” (Chinook) Indians acquired their name through shaping in infancy, as shown in a diag
 Shown here is Sigmund Freud in 1931 as he poses for a sculptor in Vienna, Austria. Although Freud ...
Shown here is Sigmund Freud in 1931 as he poses for a sculptor in Vienna, Austria. Although Freud ...