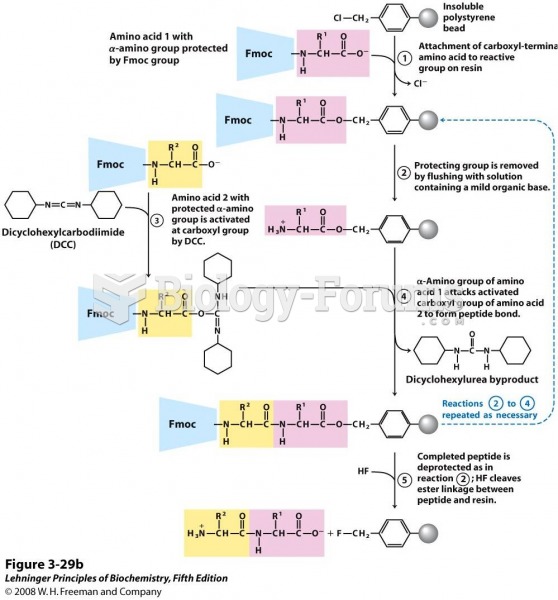
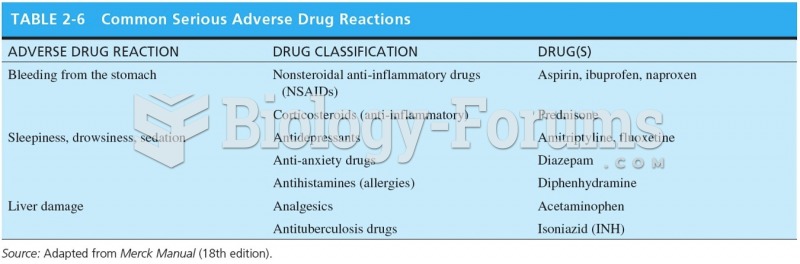
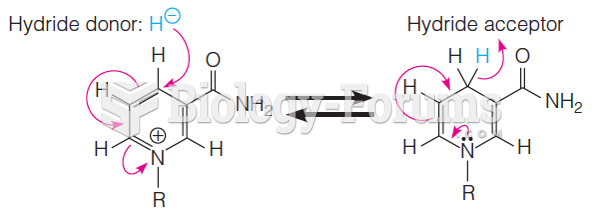
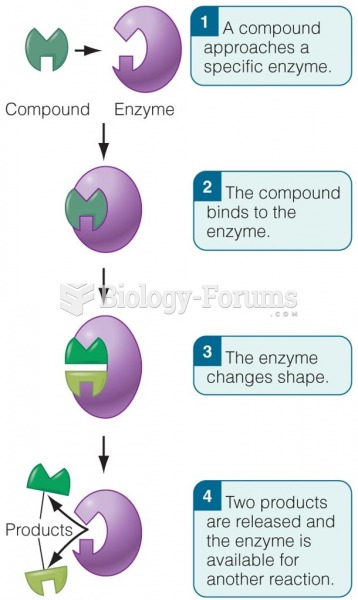
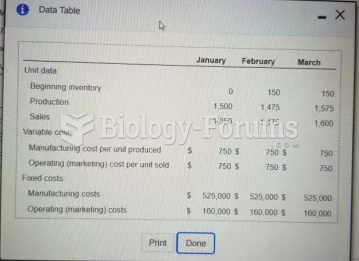
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
Though methadone is often used to treat dependency on other opioids, the drug itself can be abused. Crushing or snorting methadone can achieve the opiate "rush" desired by addicts. Improper use such as these can lead to a dangerous dependency on methadone. This drug now accounts for nearly one-third of opioid-related deaths.