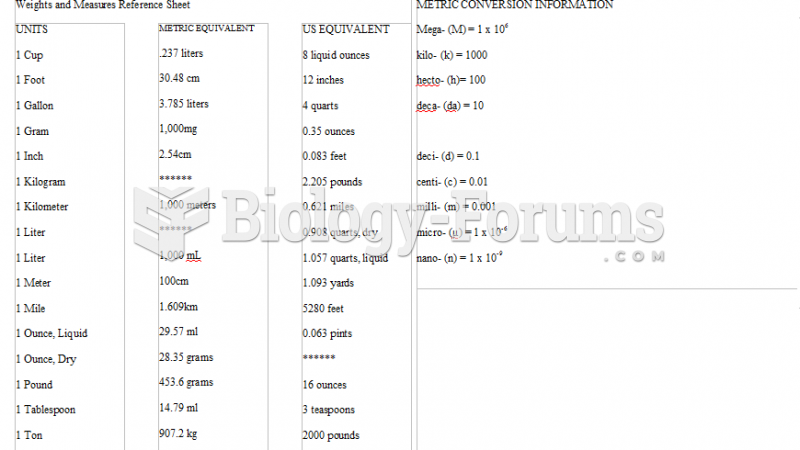
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
Did you know?
Prostaglandins were first isolated from human semen in Sweden in the 1930s. They were so named because the researcher thought that they came from the prostate gland. In fact, prostaglandins exist and are synthesized in almost every cell of the body.