|
|
|
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
 Shown here is Sigmund Freud in 1931 as he poses for a sculptor in Vienna, Austria. Although Freud ...
Shown here is Sigmund Freud in 1931 as he poses for a sculptor in Vienna, Austria. Although Freud ...
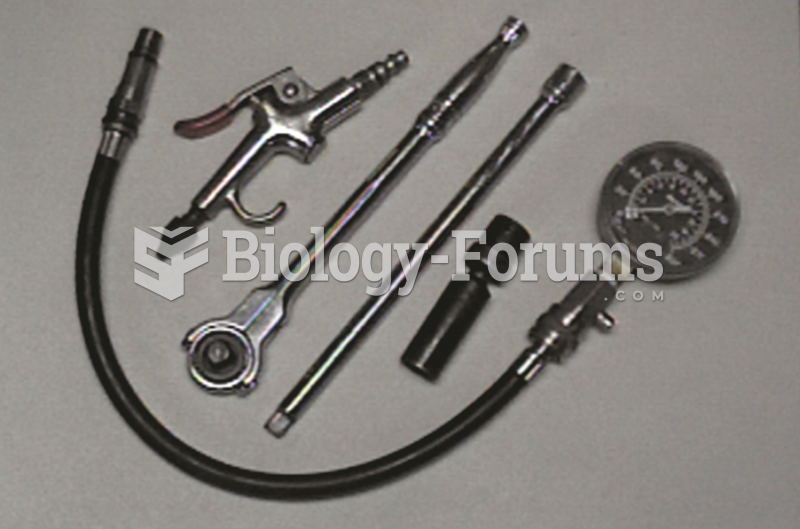 The tools and equipment needed to perform a compression test include a compression gauge, an air ...
The tools and equipment needed to perform a compression test include a compression gauge, an air ...