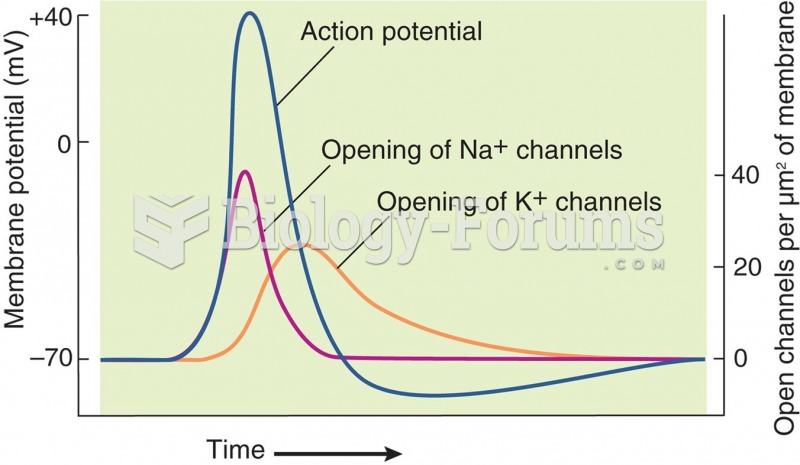
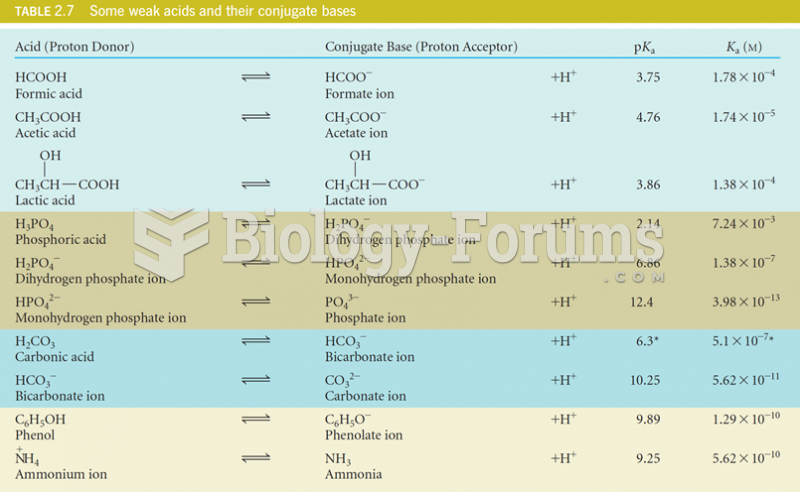
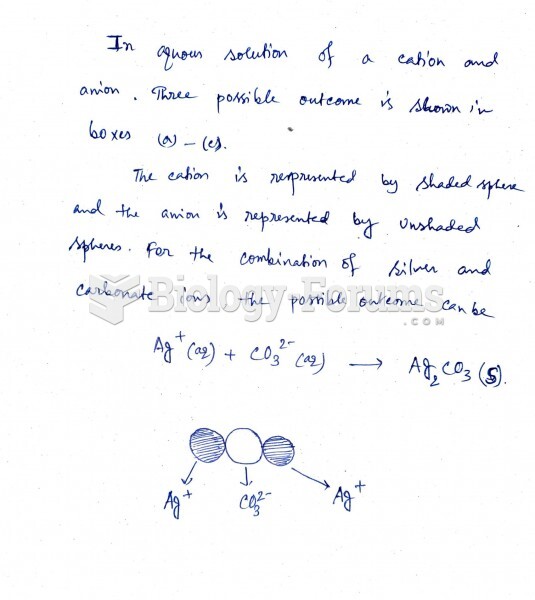
|
|
|
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Excessive alcohol use costs the country approximately $235 billion every year.
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.