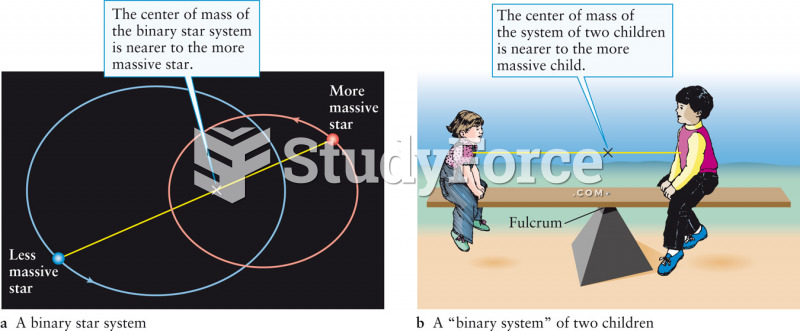
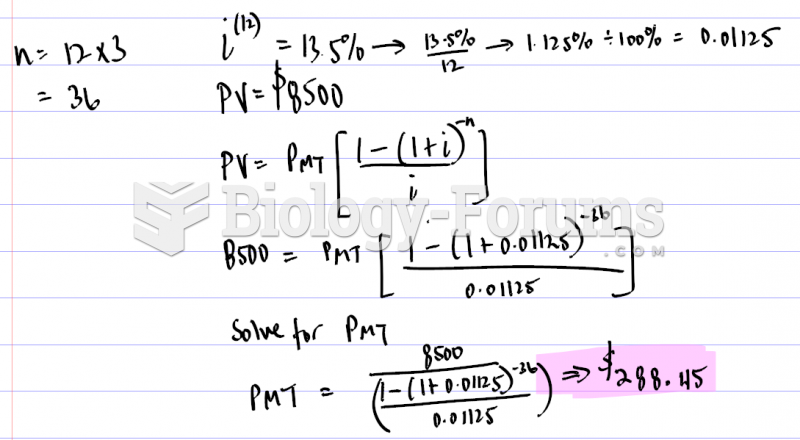
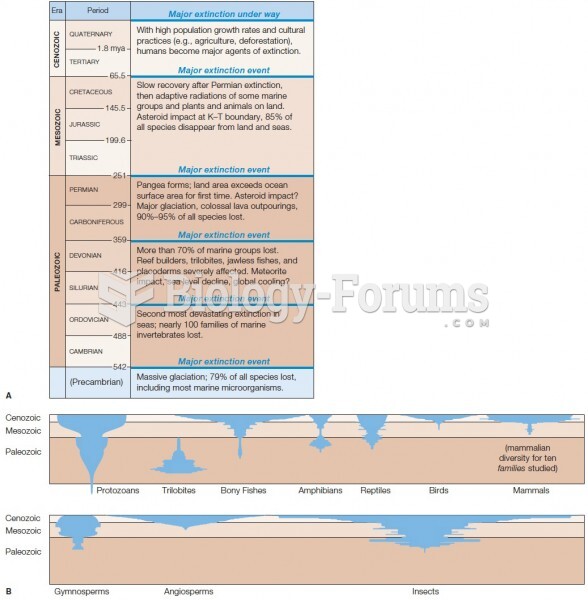
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Did you know?
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.