|
|
|
People about to have surgery must tell their health care providers about all supplements they take.
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
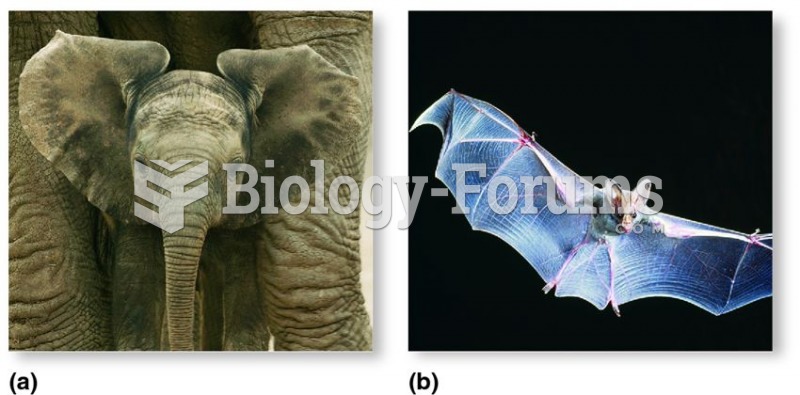
If you could remove all of your skin, it would weigh up to 5 pounds.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.