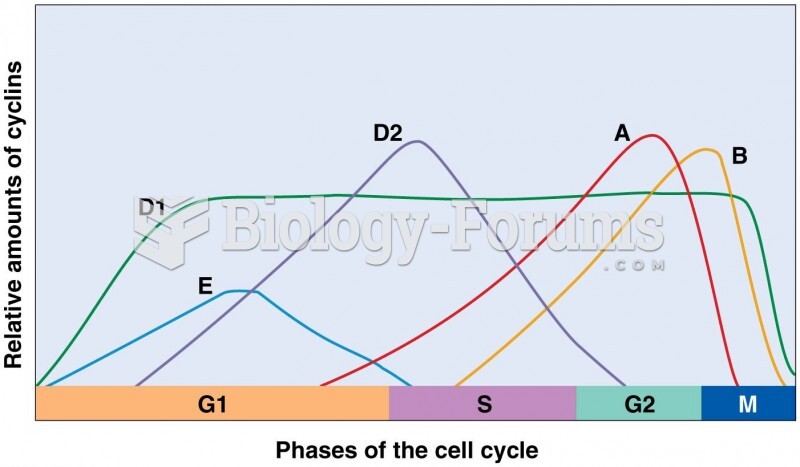
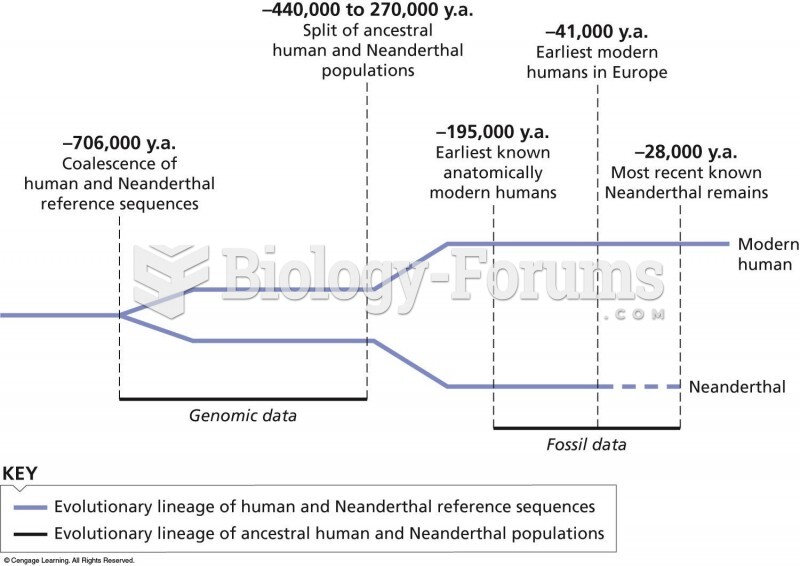
|
|
|
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
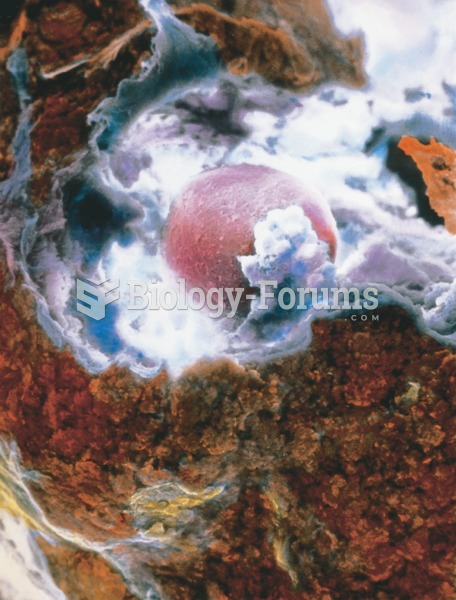 scanning electron micrograph showing an ovum (pink) released by the ovary at ovulation surrounded by
scanning electron micrograph showing an ovum (pink) released by the ovary at ovulation surrounded by
 A passenger train crosses Stony Creek Bridge in the Rocky Mountains in 1878. Railroads were importan
A passenger train crosses Stony Creek Bridge in the Rocky Mountains in 1878. Railroads were importan