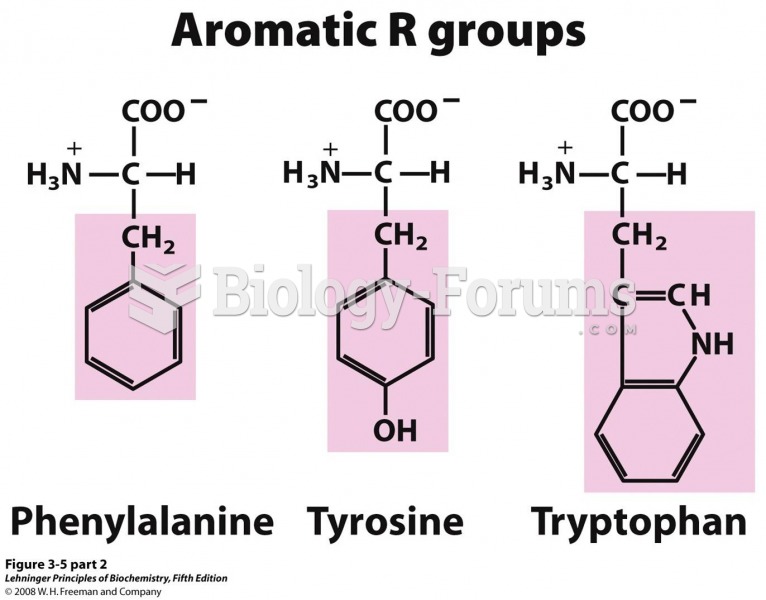
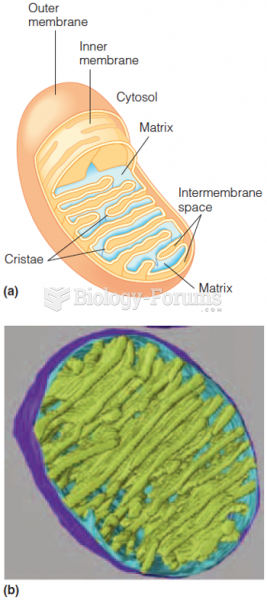
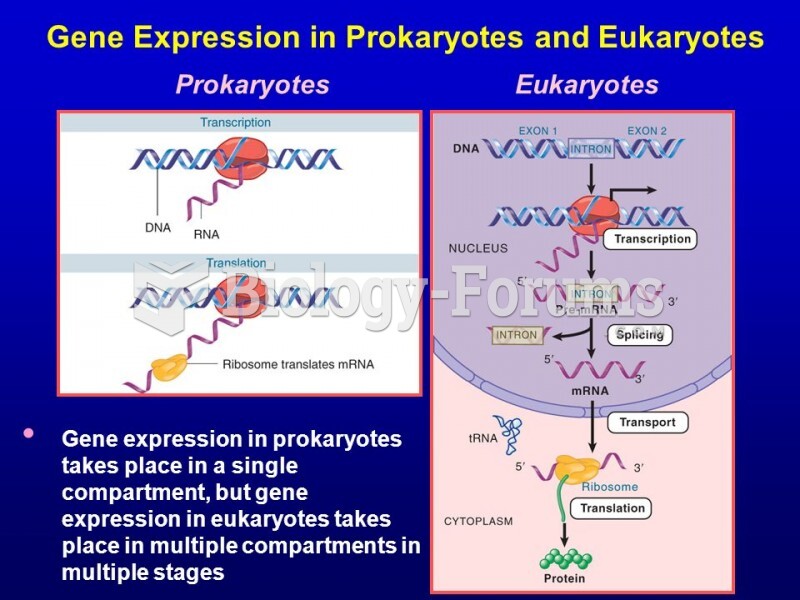
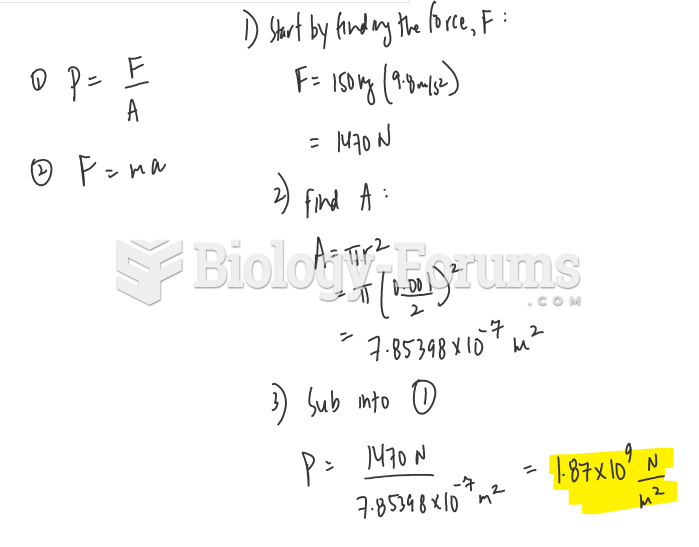
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.