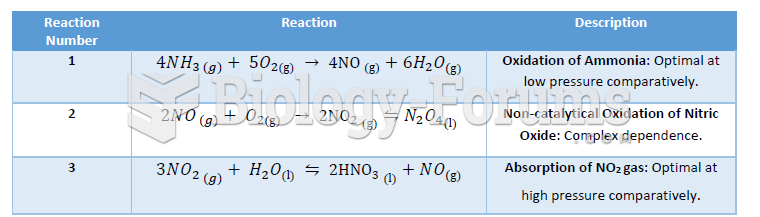
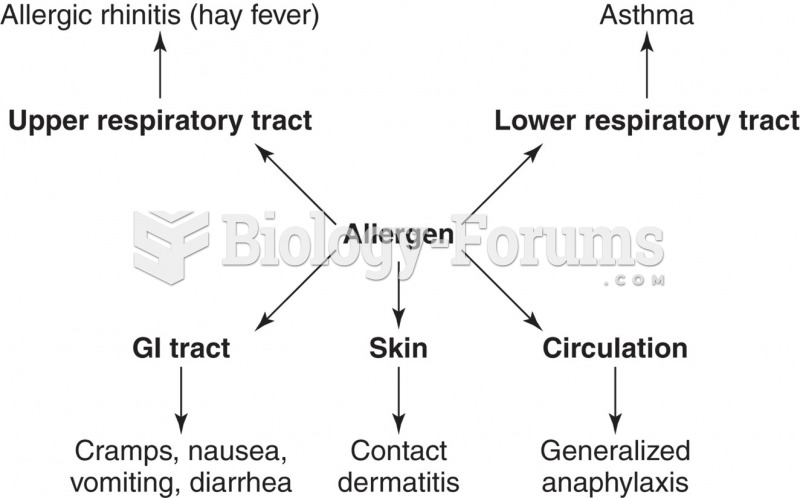
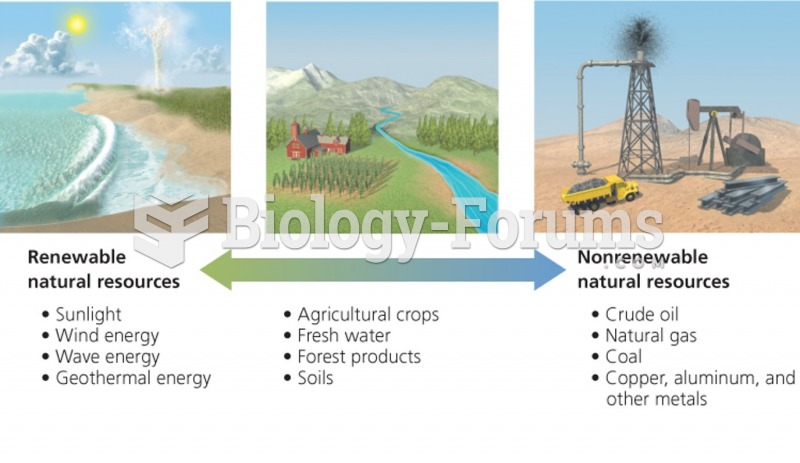
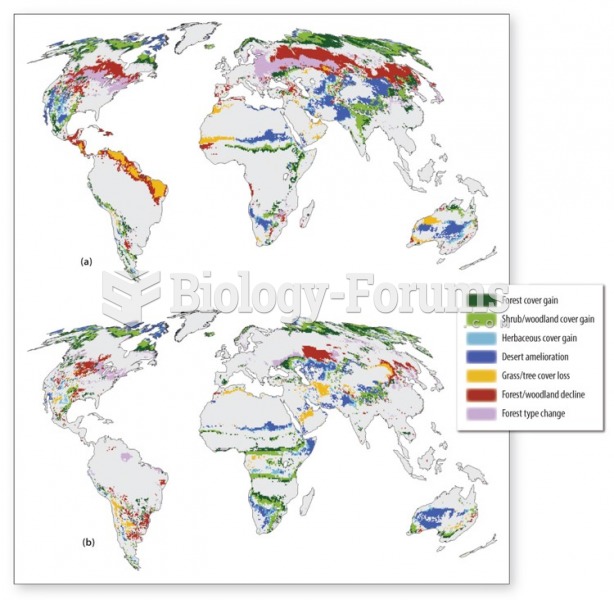
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.