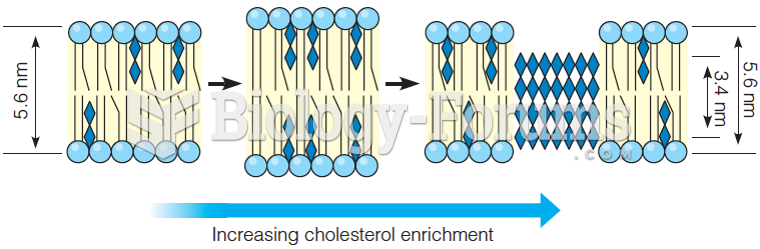
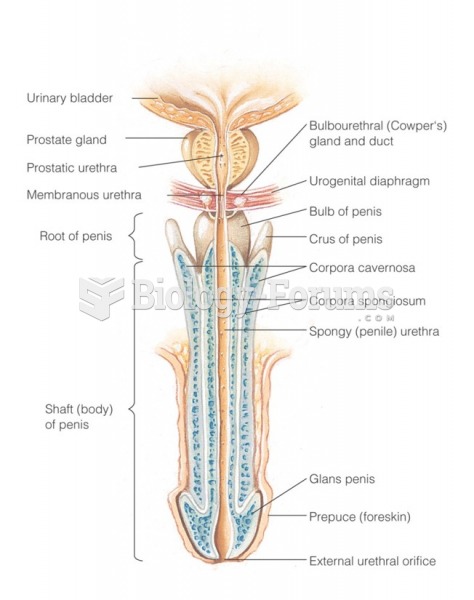
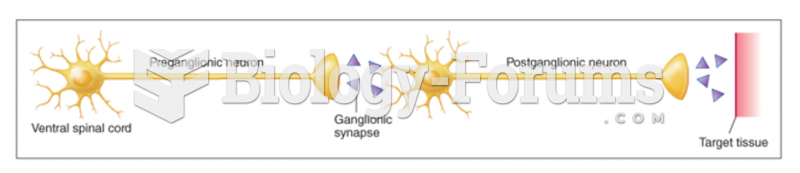
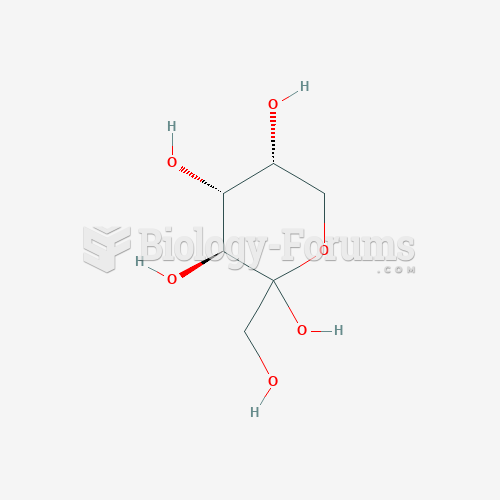
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.