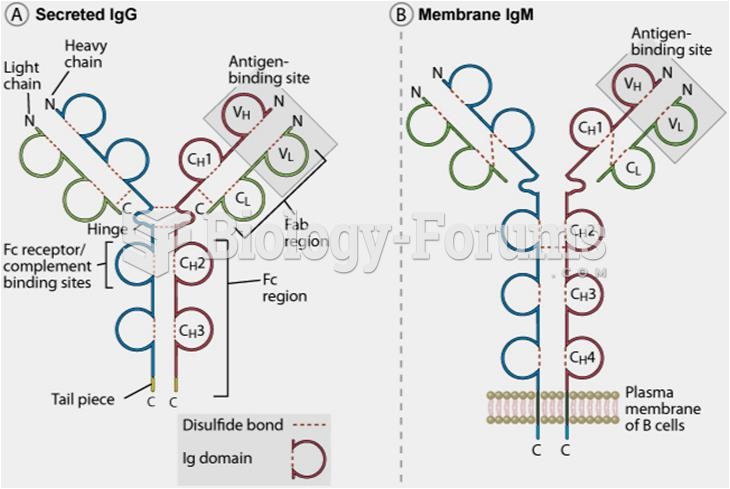
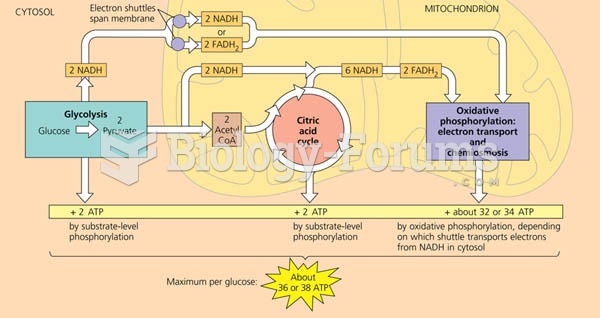
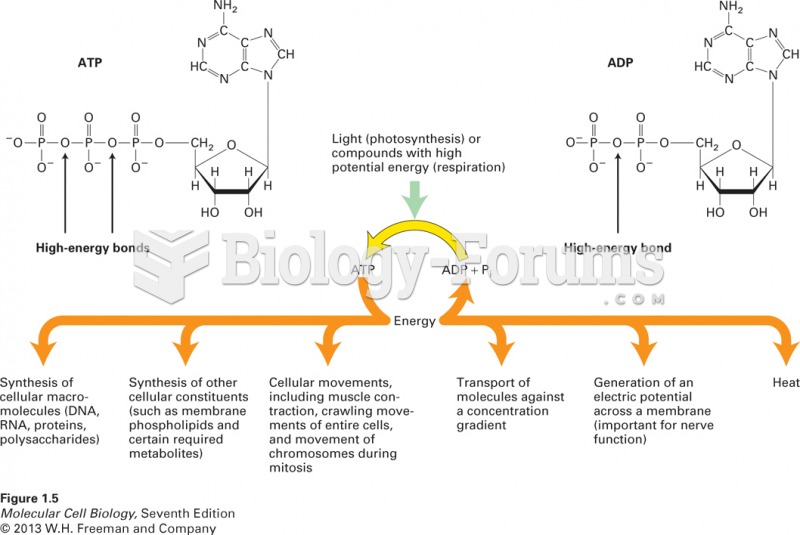
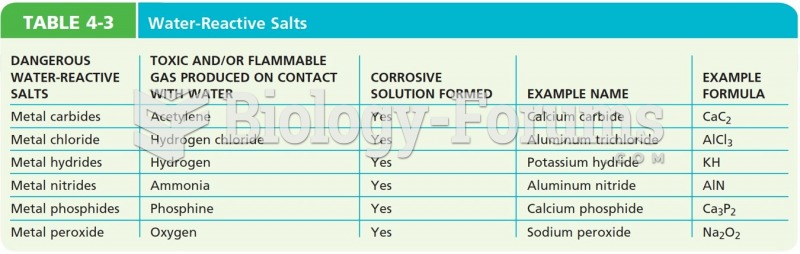
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.