This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
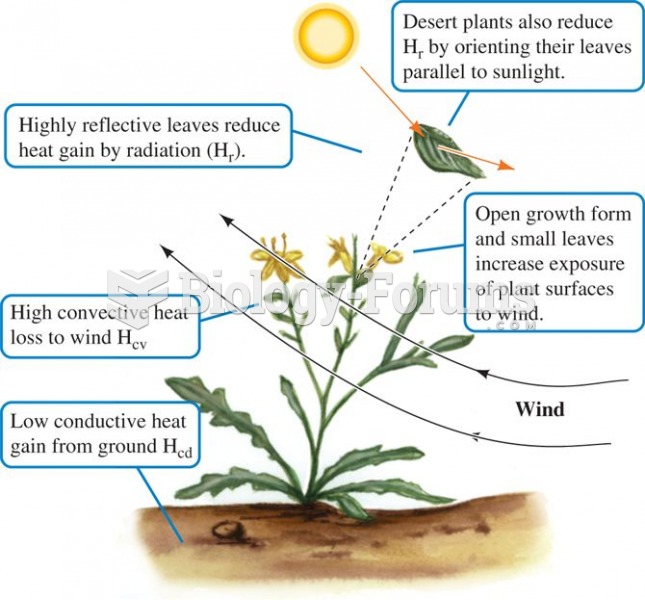 The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
 Place sheet or towel over area to be heated. Use sufficient heat barrier for the heat source used. ...
Place sheet or towel over area to be heated. Use sufficient heat barrier for the heat source used. ...