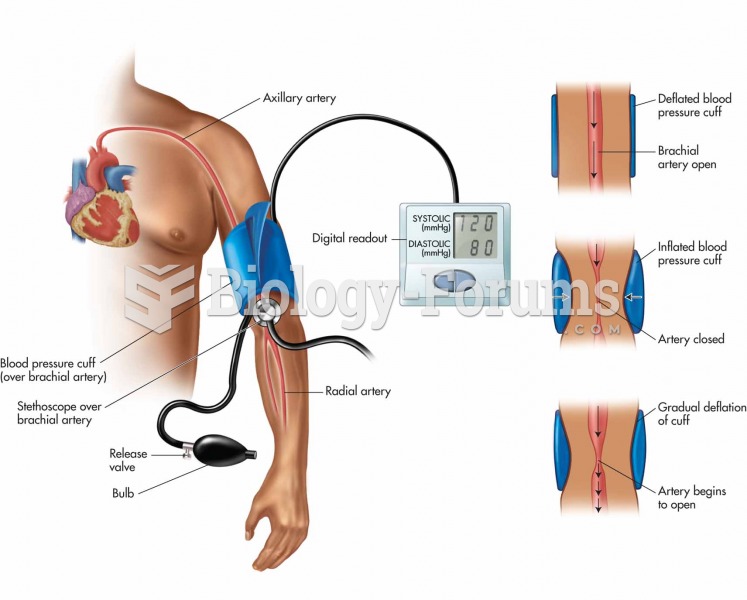
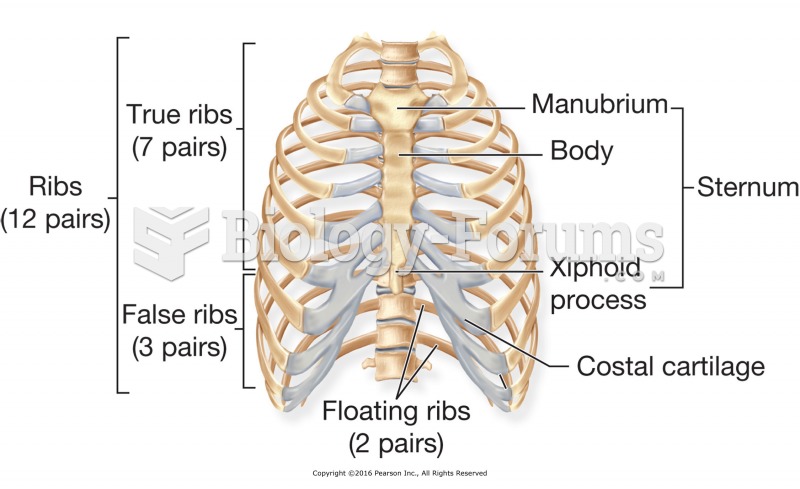
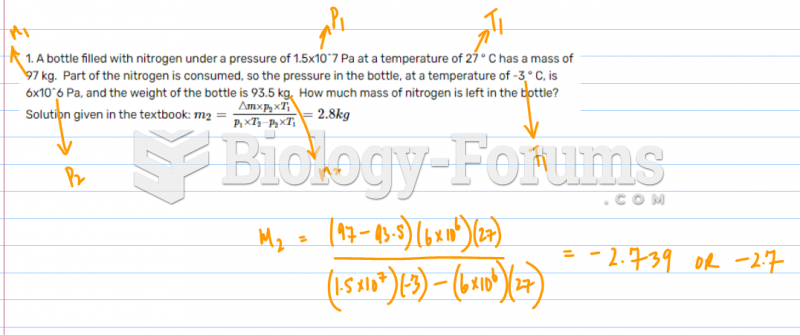
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
Did you know?
Approximately one in three babies in the United States is now delivered by cesarean section. The number of cesarean sections in the United States has risen 46% since 1996.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.