This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
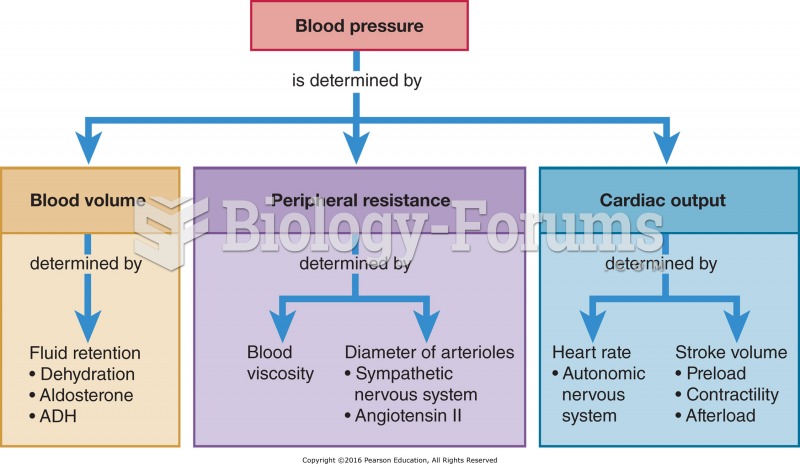
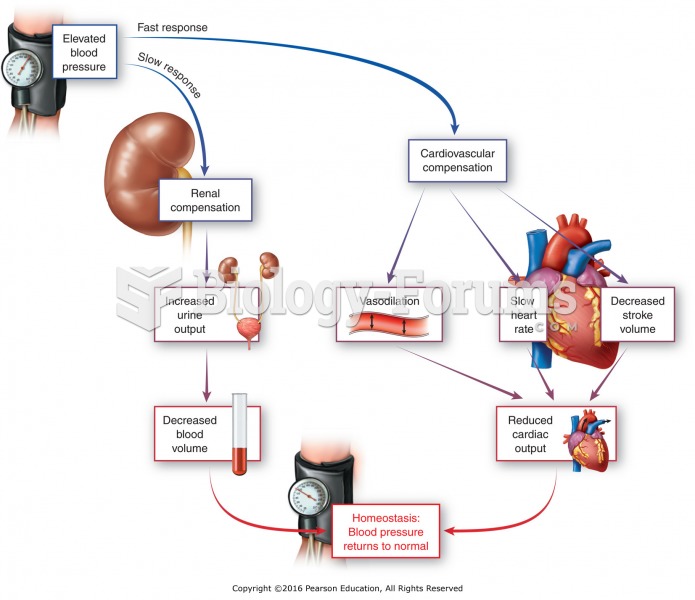
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.