|
|
|
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
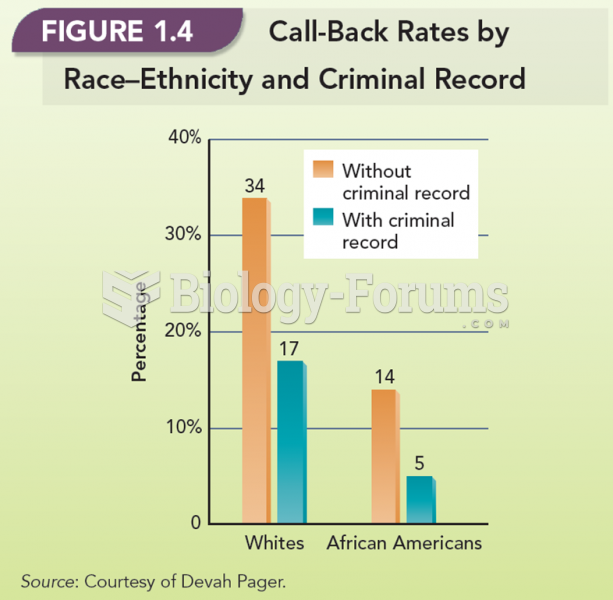 In a project on job discrimination, Pager prepared identical résumés for the teams, but with one ...
In a project on job discrimination, Pager prepared identical résumés for the teams, but with one ...
 Apply lubricant to the entire right limb using basic sliding effleurage. Apply moderate pressure ...
Apply lubricant to the entire right limb using basic sliding effleurage. Apply moderate pressure ...