|
|
|
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
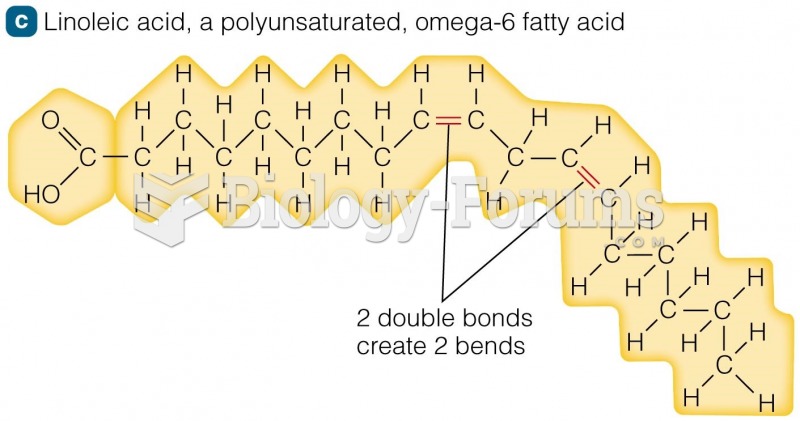
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.