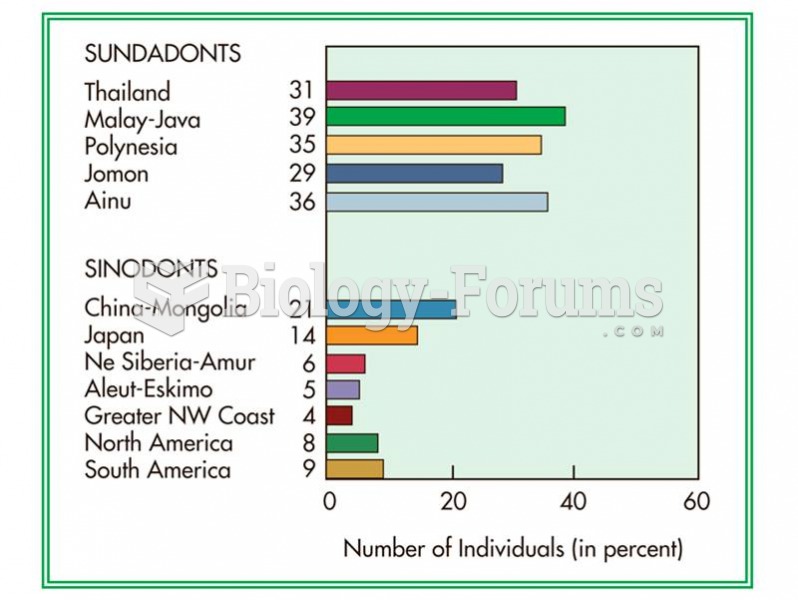
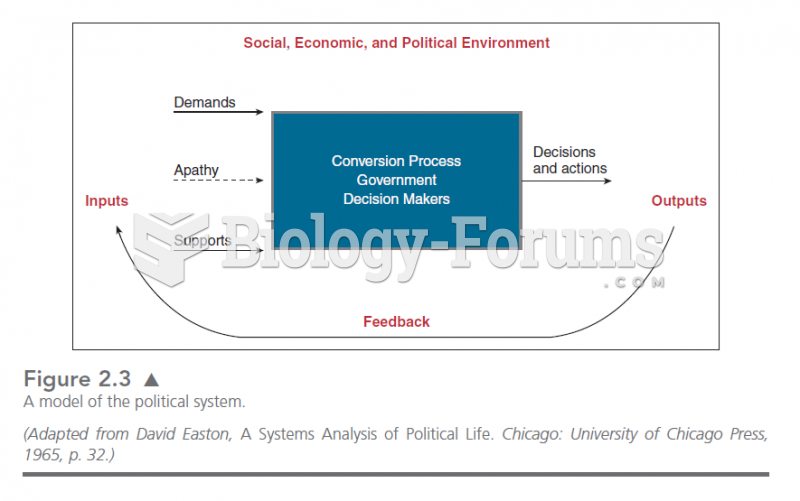
|
|
|
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
Certain topical medications such as clotrimazole and betamethasone are not approved for use in children younger than 12 years of age. They must be used very cautiously, as directed by a doctor, to treat any child. Children have a much greater response to topical steroid medications.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
 Some playgrounds near streets with heavy traffic may still have high levels of lead from gasoline ...
Some playgrounds near streets with heavy traffic may still have high levels of lead from gasoline ...
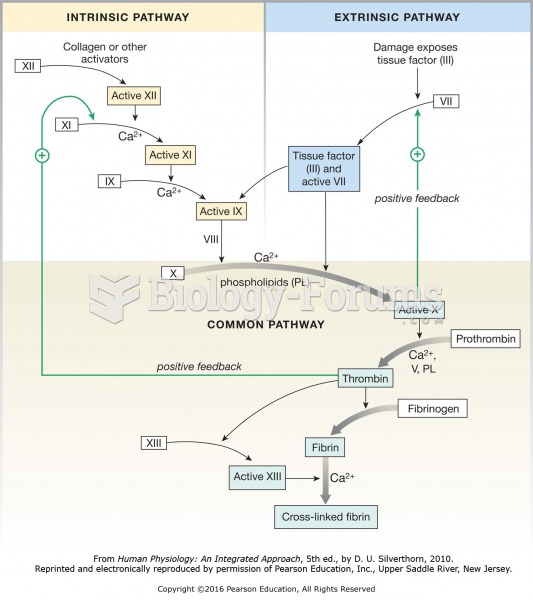 The coagulation cascade. Both the intrinsic pathway and extrinsic pathway lead to a common pathway ...
The coagulation cascade. Both the intrinsic pathway and extrinsic pathway lead to a common pathway ...