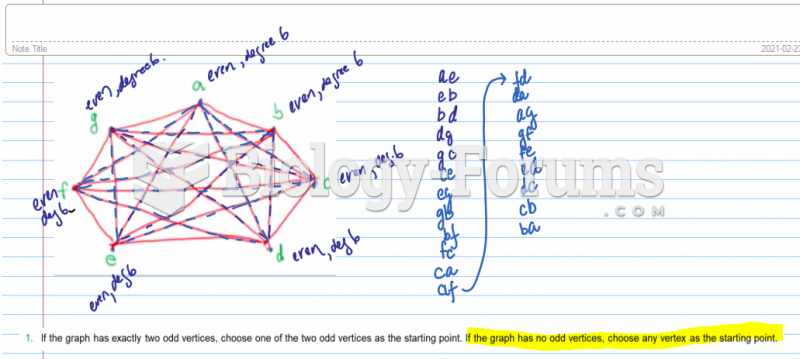
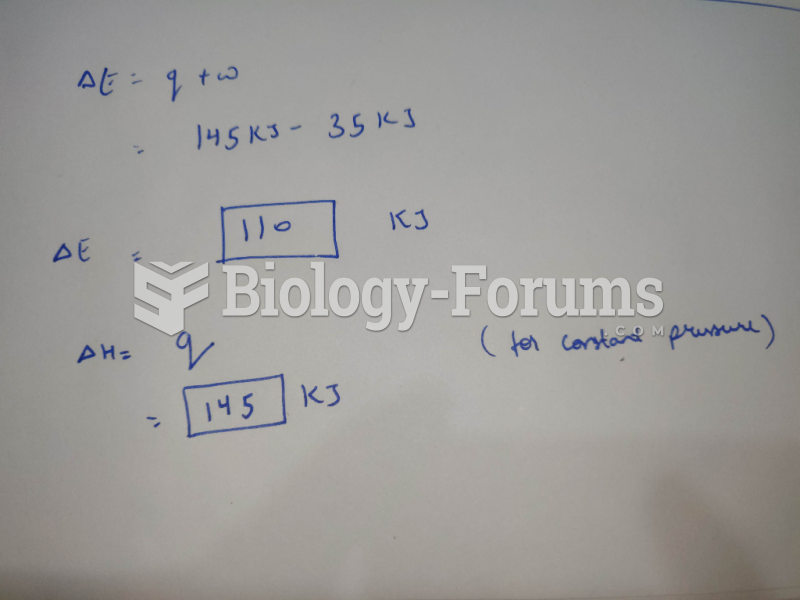
|
|
|
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Although puberty usually occurs in the early teenage years, the world's youngest parents were two Chinese children who had their first baby when they were 8 and 9 years of age.
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.