|
|
|
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
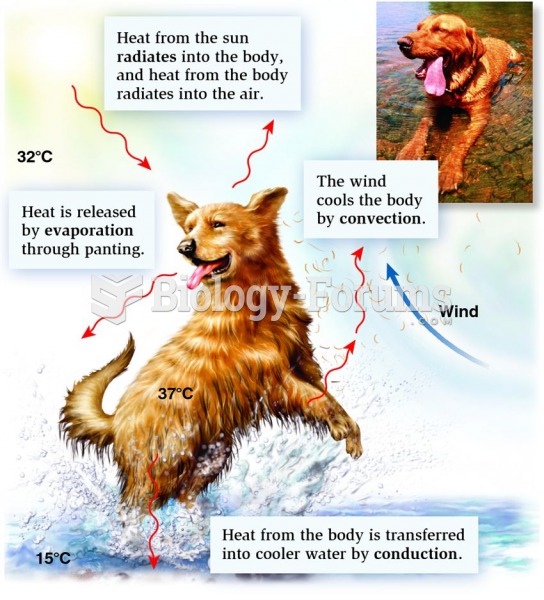 The four ways in which animals exchange heat with the environment are radiation, evaporation, convec
The four ways in which animals exchange heat with the environment are radiation, evaporation, convec
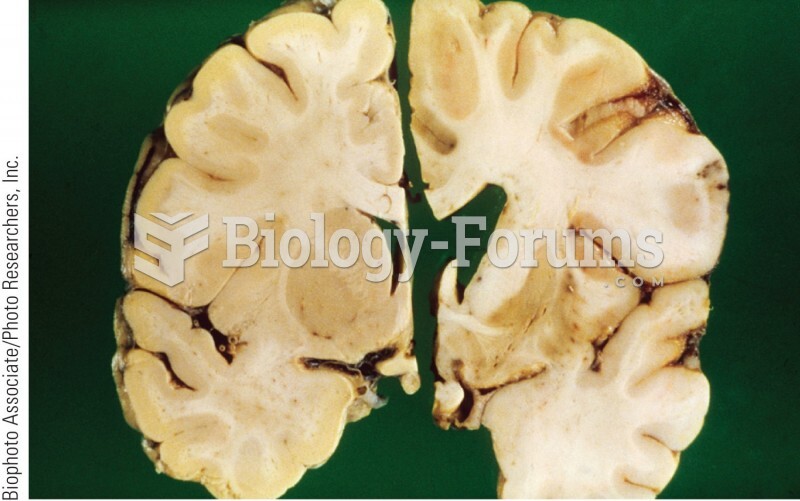
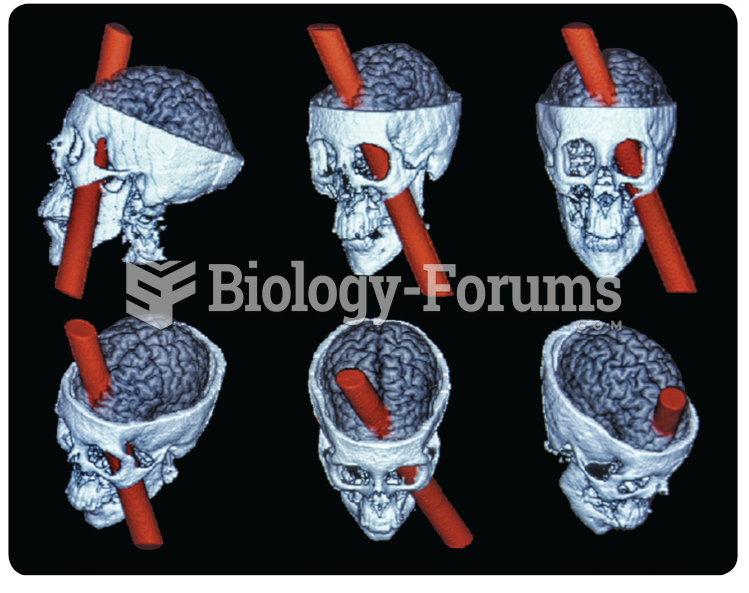 A reconstruction of the brain injury of Phineas Gage. The damage focused on the medial prefrontal ...
A reconstruction of the brain injury of Phineas Gage. The damage focused on the medial prefrontal ...
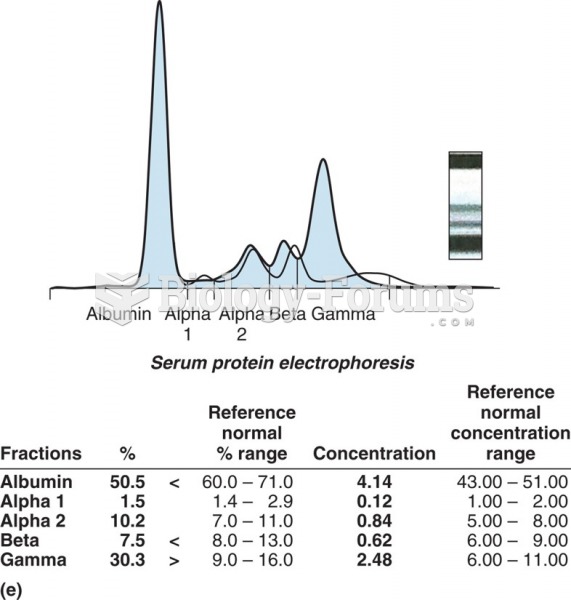 Electrophoresis gel in a densitometer. SPE densitometry showing an M spike in the gamma region with ...
Electrophoresis gel in a densitometer. SPE densitometry showing an M spike in the gamma region with ...