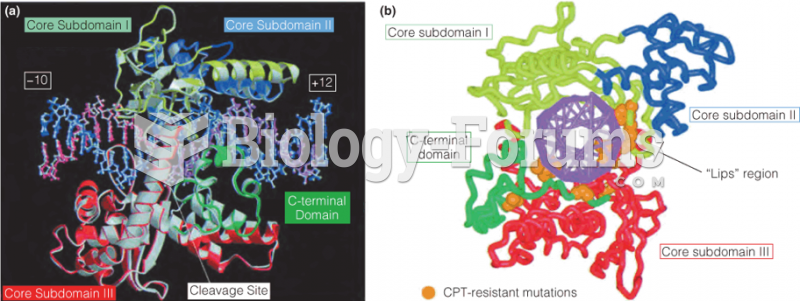
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Did you know?
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
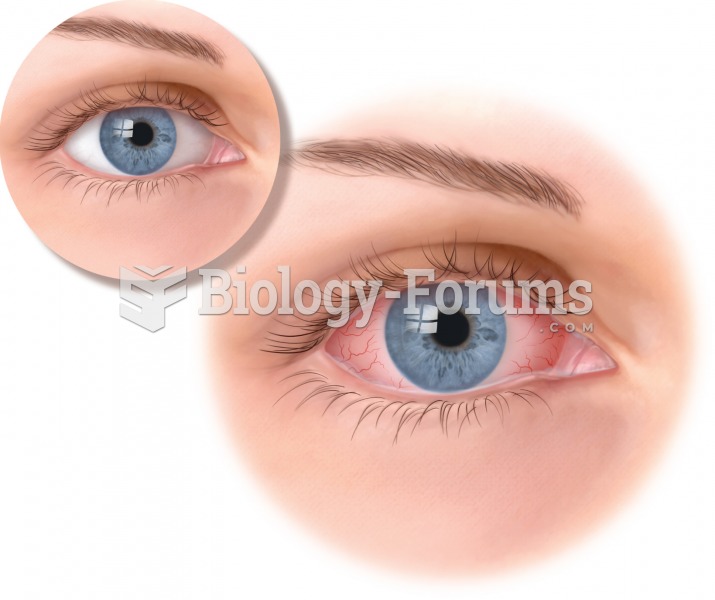 Conjunctivitis, with the characteristic “pinkeye” appearance. A yellow crusty exudate is also common
Conjunctivitis, with the characteristic “pinkeye” appearance. A yellow crusty exudate is also common
 A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh
A the subfossil lemurs of Madagascar filled a variety of niches occupied elsewhere by monkeys, as sh