This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
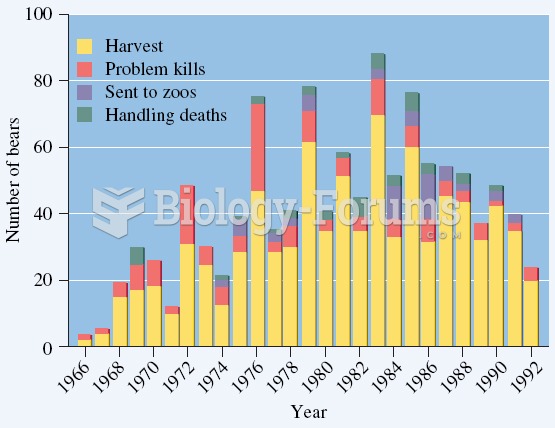 Over nearly 30 years, humans removed many polar bears for a variety of reasons, from a population in
Over nearly 30 years, humans removed many polar bears for a variety of reasons, from a population in
 Critical thinking involves analysis in which the nurse examines patient data available from a variet
Critical thinking involves analysis in which the nurse examines patient data available from a variet