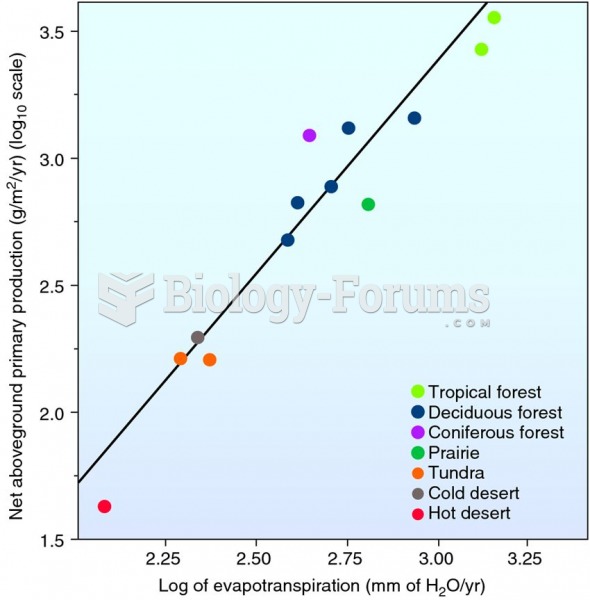
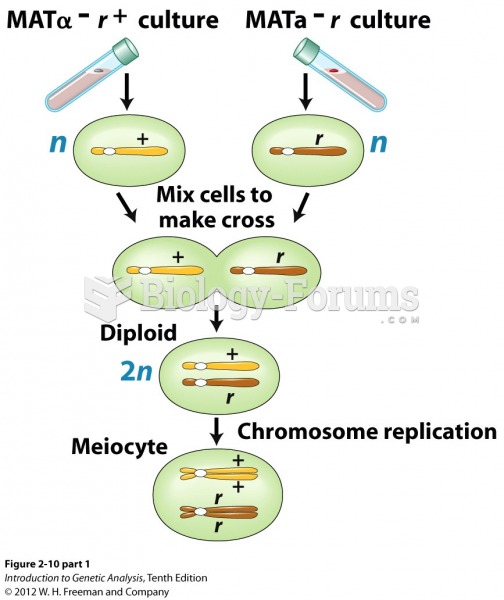
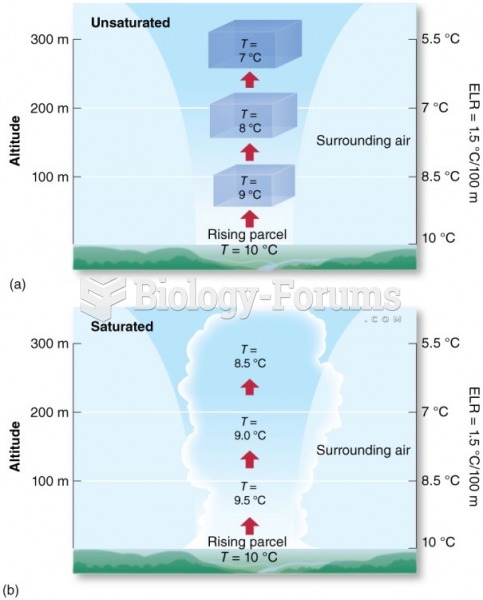
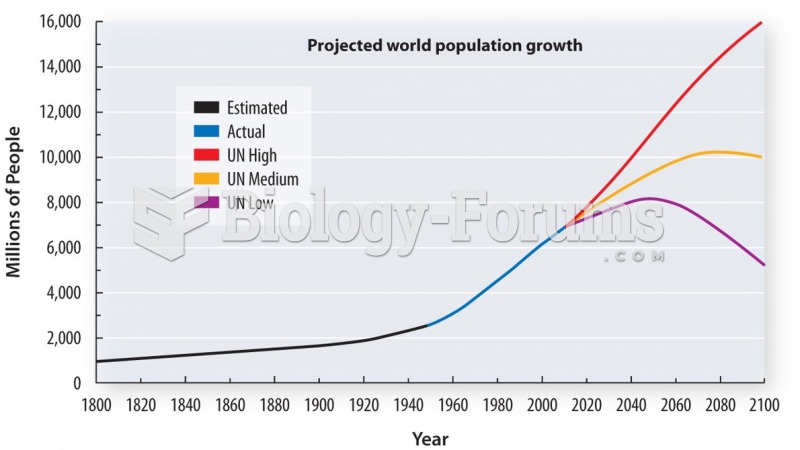
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Computer programs are available that crosscheck a new drug's possible trade name with all other trade names currently available. These programs detect dangerous similarities between names and alert the manufacturer of the drug.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.