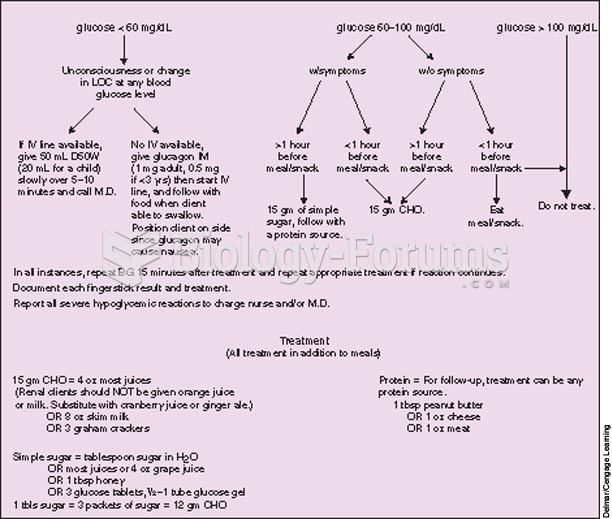
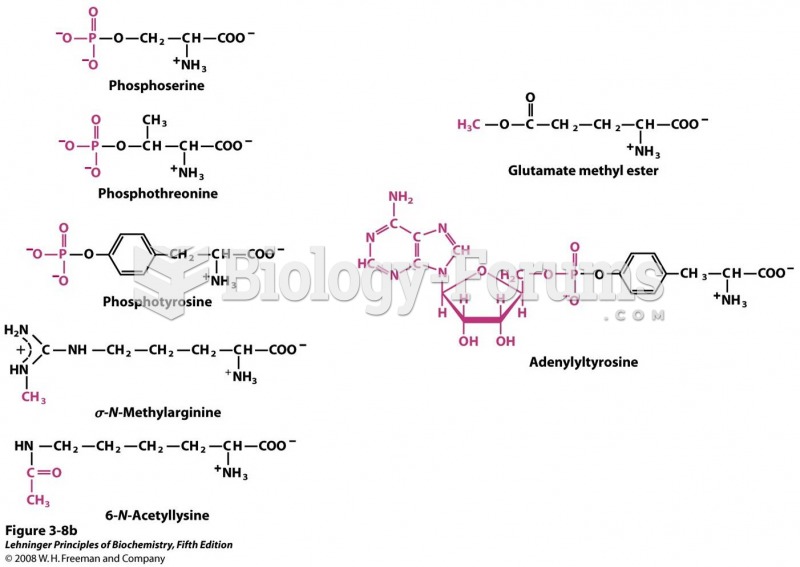
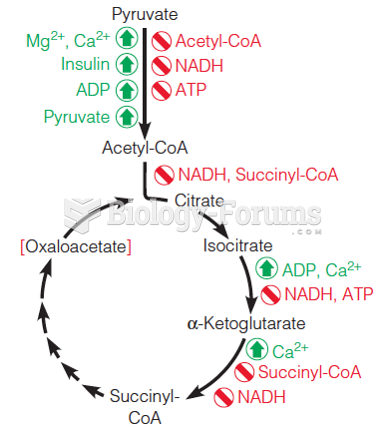
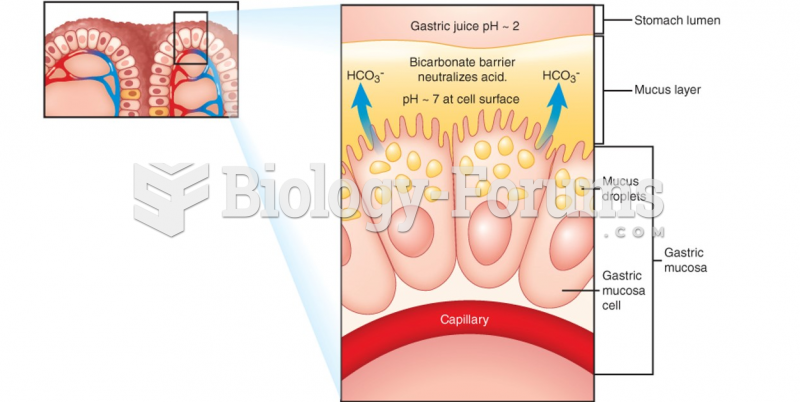
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.