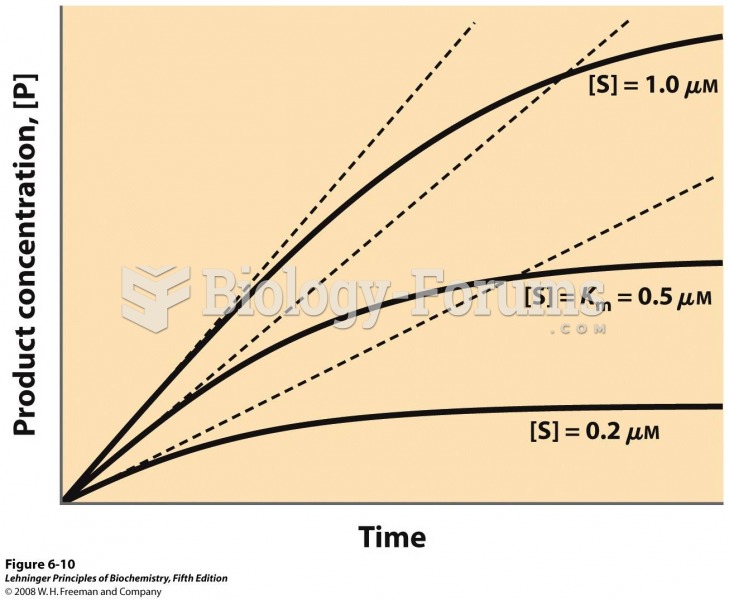
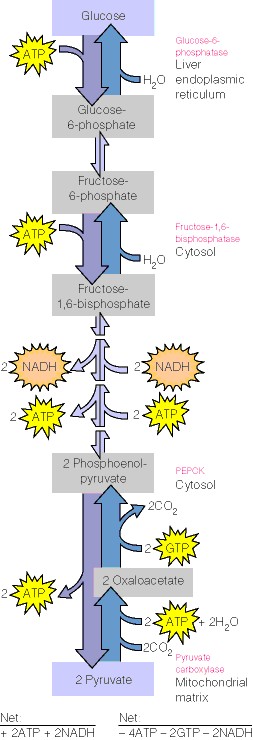
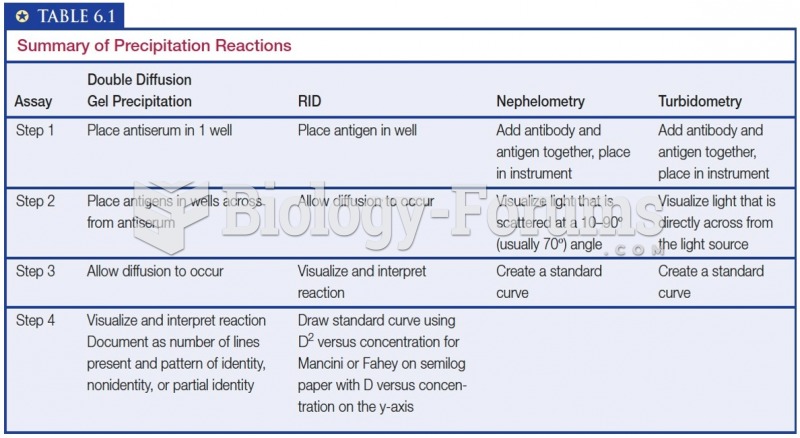
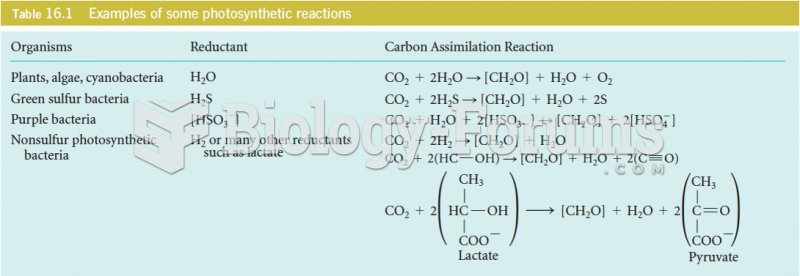
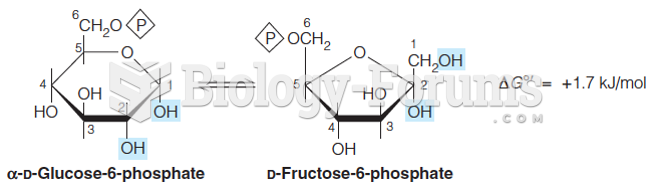
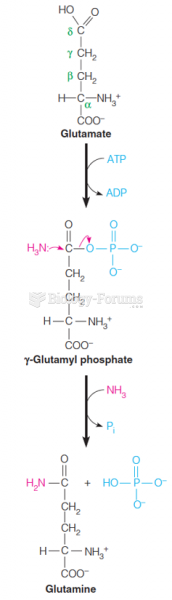
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.