|
|
|
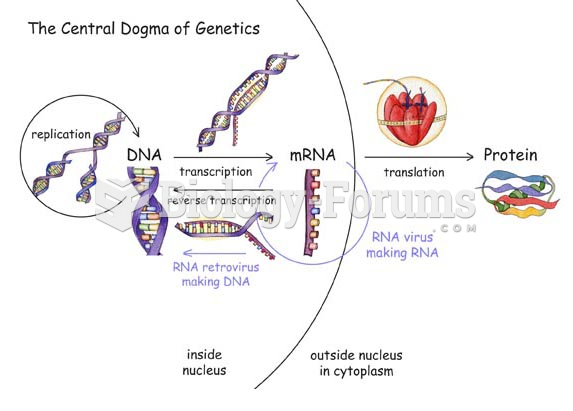
Though Candida and Aspergillus species are the most common fungal pathogens causing invasive fungal disease in the immunocompromised, infections due to previously uncommon hyaline and dematiaceous filamentous fungi are occurring more often today. Rare fungal infections, once accurately diagnosed, may require surgical debridement, immunotherapy, and newer antifungals used singly or in combination with older antifungals, on a case-by-case basis.
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.