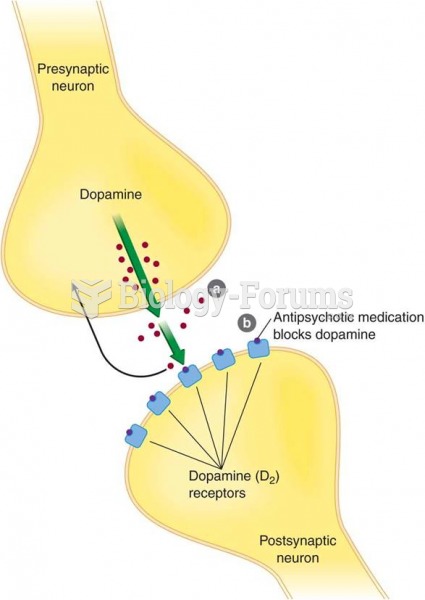
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
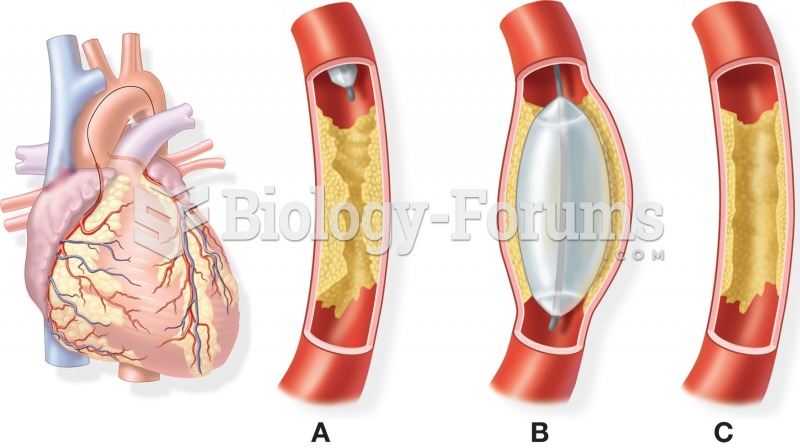 Balloon angioplasty: (A) deflated balloon catheter is approaching an atherosclerotic plaque; (B) pla
Balloon angioplasty: (A) deflated balloon catheter is approaching an atherosclerotic plaque; (B) pla
 An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution
An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution