|
|
|
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Although puberty usually occurs in the early teenage years, the world's youngest parents were two Chinese children who had their first baby when they were 8 and 9 years of age.
GI conditions that will keep you out of the U.S. armed services include ulcers, varices, fistulas, esophagitis, gastritis, congenital abnormalities, inflammatory bowel disease, enteritis, colitis, proctitis, duodenal diverticula, malabsorption syndromes, hepatitis, cirrhosis, cysts, abscesses, pancreatitis, polyps, certain hemorrhoids, splenomegaly, hernias, recent abdominal surgery, GI bypass or stomach stapling, and artificial GI openings.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.
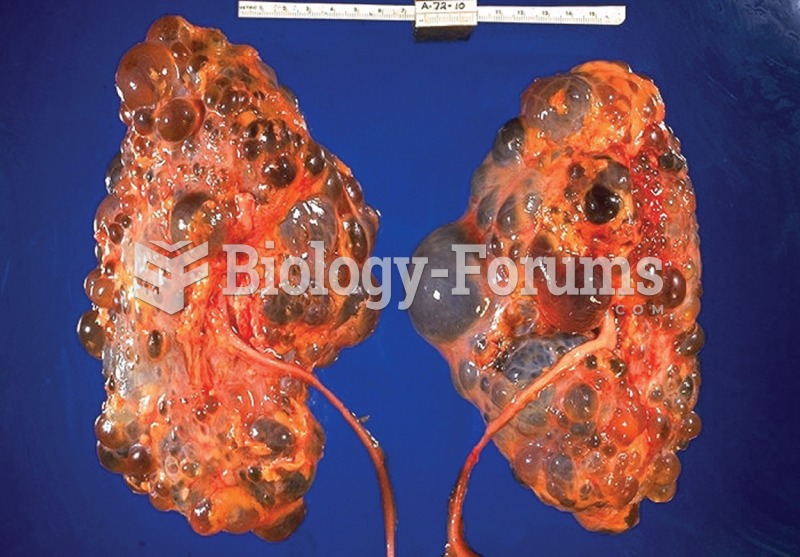 Polycystic kidney disease. Notice the presence of numerous fluid-filled sacs, or cysts, in these kid
Polycystic kidney disease. Notice the presence of numerous fluid-filled sacs, or cysts, in these kid
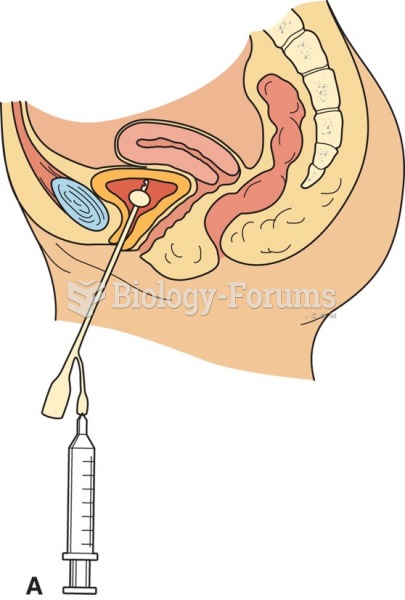 Foley catheter: The inflated balloon at the tip of the catheter holds the Foley catheter in place in ...
Foley catheter: The inflated balloon at the tip of the catheter holds the Foley catheter in place in ...
 Not only was the housing filled with nuts, but also this air filter was extremely dirty, indicating ...
Not only was the housing filled with nuts, but also this air filter was extremely dirty, indicating ...