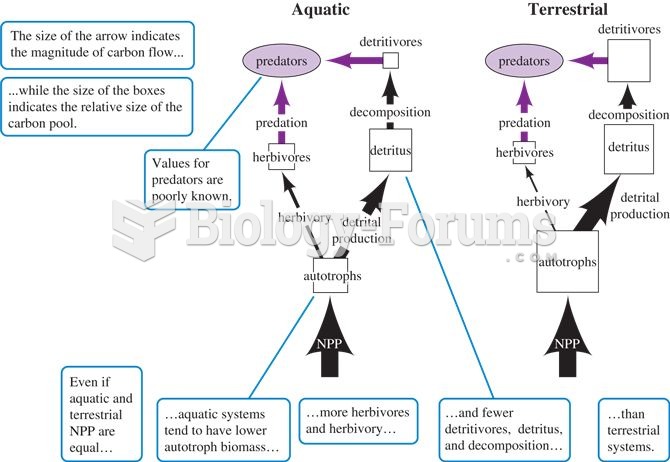
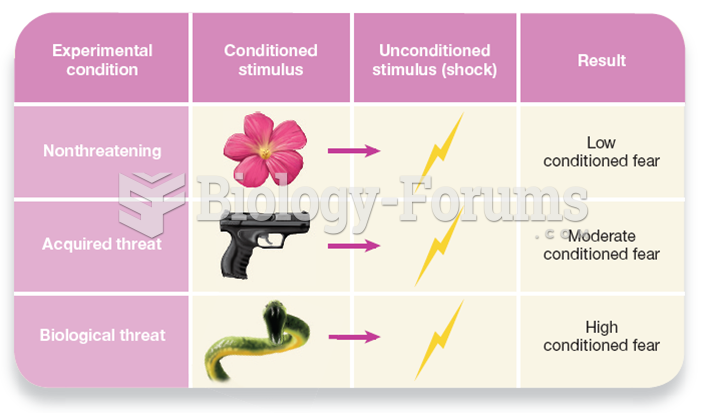
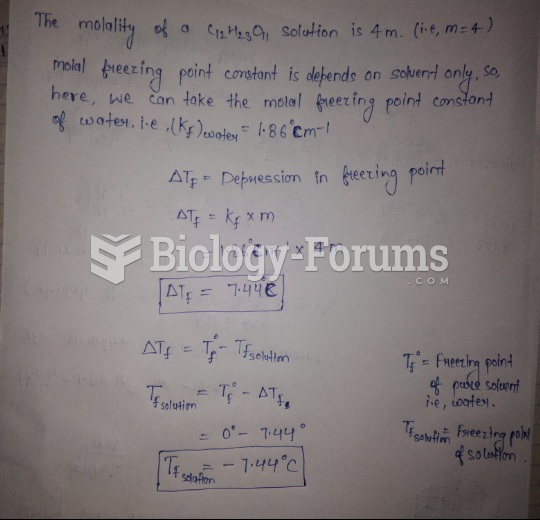
|
|
|
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.