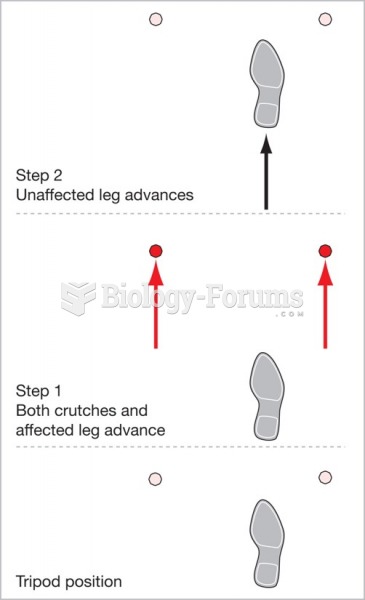
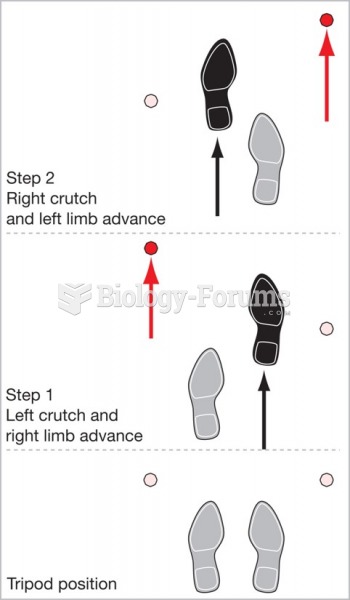
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Inotropic therapy does not have a role in the treatment of most heart failure patients. These drugs can make patients feel and function better but usually do not lengthen the predicted length of their lives.
Did you know?
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.