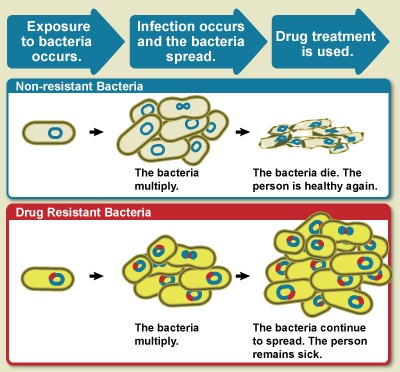
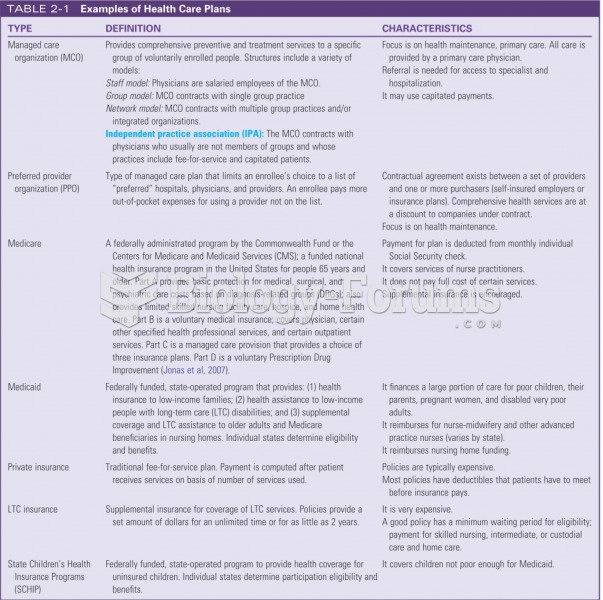
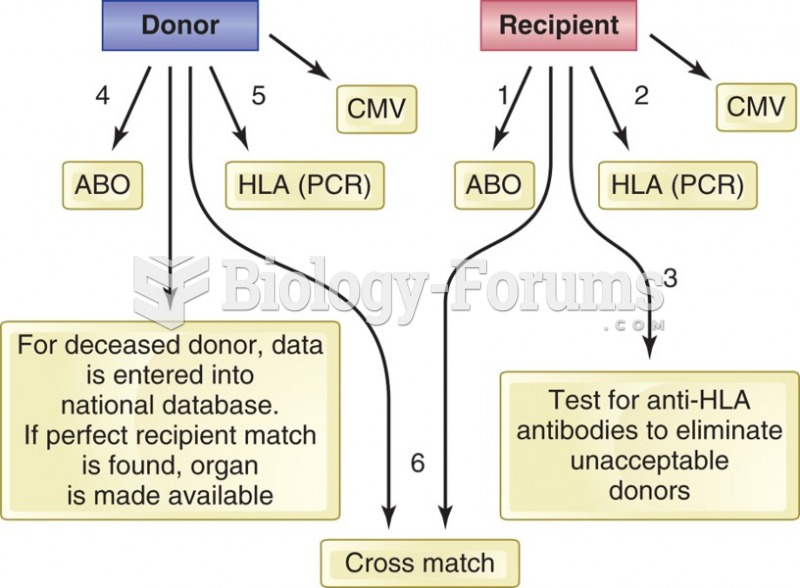
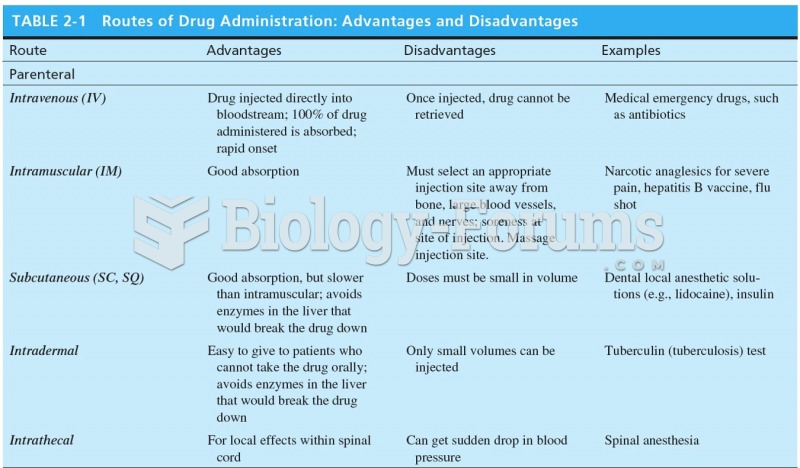
Which of the following is not subject to mandatory bargaining?
a. drug testing
b. retirement plans c. holidays
d. no-strike clauses
e. all are subject to mandatory bargaining
Question 2
In 1999, the Drugs-R-Us began testing its new drug, Reduceo, a medicine to help people lose weight. Tests looked promising and, in 2006, the company applied to the FDA for approval to market Reduceo as a prescription drug. In March 2009, the FDA granted Drug-R-Us approval to market Reduceo. Reduceo was sold with some diet enhancing cookies that contained no drugs but were claimed to help dieting with Reduceo. Frank saw an ad for the new drug. The Reduceo ad stated that it was a wonder drug and tests prove it is the safest weight reduction drug on the market today Frank was interested and made an appointment to see his doctor. Frank's physician prescribed the new drug for his patient. Frank had no success using other weight-loss drugs, and dieting and exercise seemed ineffective. Frank took Reduceo from June until the end of August and lost 25 lbs. He also ate Reduceo's cookies. He was delighted with his weight loss, but was concerned because dots appeared before his eyes, causing disorientation. One day, the dots appeared before Frank's eyes while he was driving. He became disoriented and hit a tree and was seriously injured. He sued Drugs-R-Us, alleging negligence in manufacturing and inadequate warning of possible effects, as well as for deceptive advertising. If it turns out that Frank had a vision problem before he started taking Reduceo, and the instructions with the drug told doctors not to prescribe it in such cases, Drugs-R-Us:
a. would still be likely to be liable to Frank for his injuries as strict liability applies
b. would still be liable to Frank for his injuries if it could be shown that the firm was negligent in the way it prepared the warning statement
c. would probably not be liable due to the learned intermediary doctrine
d. would not be liable due to FDA approval which shields the drug maker e. none of the other choices