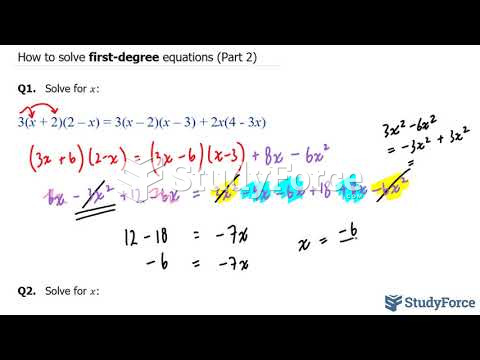
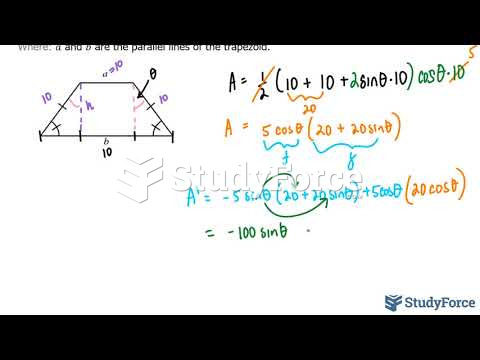
|
|
|
Green tea is able to stop the scent of garlic or onion from causing bad breath.
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.