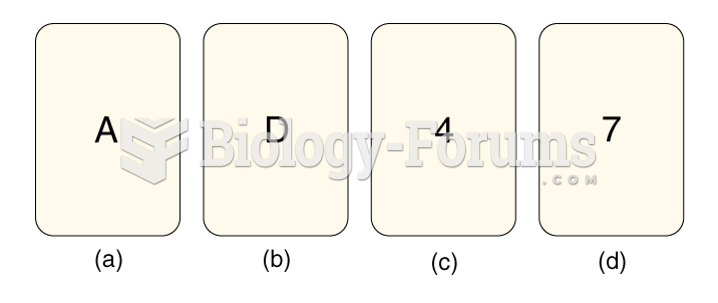
|
|
|
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Did you know?
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Did you know?
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.