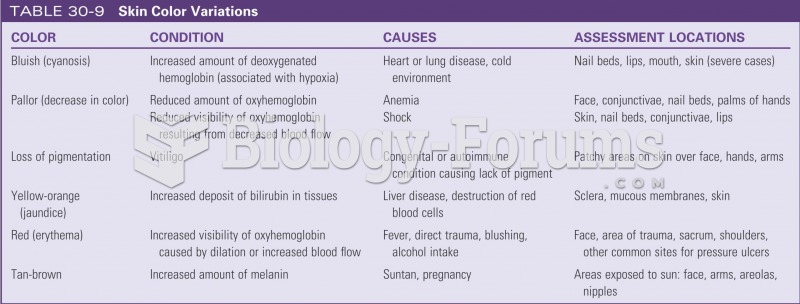
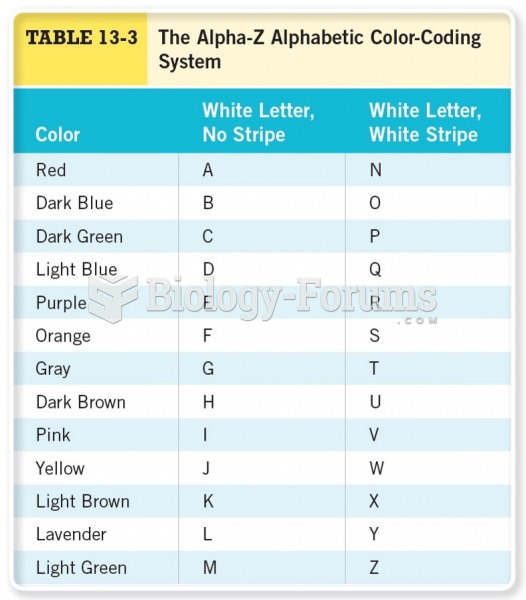
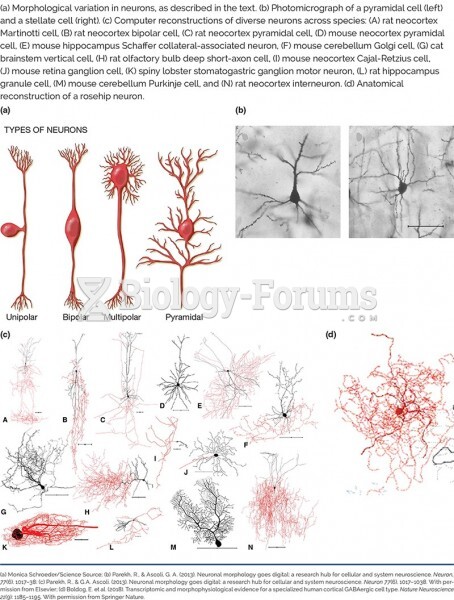
|
|
|
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
When intravenous medications are involved in adverse drug events, their harmful effects may occur more rapidly, and be more severe than errors with oral medications. This is due to the direct administration into the bloodstream.
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.