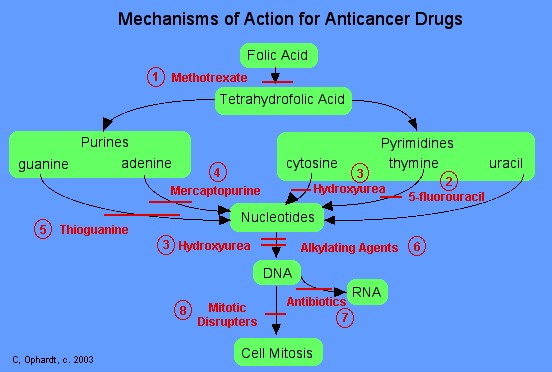
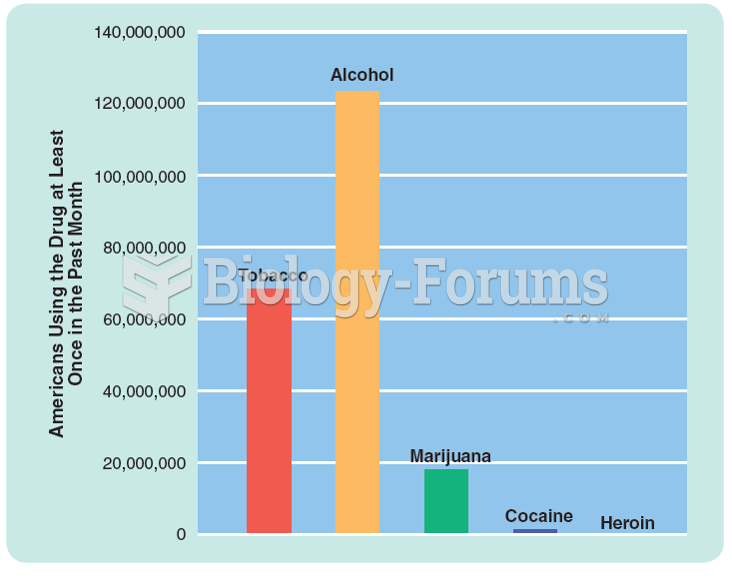
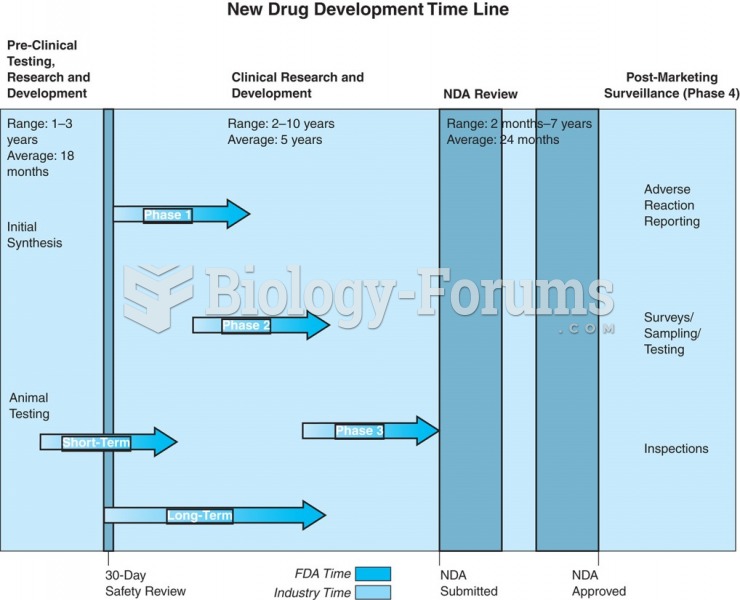
|
|
|
About 60% of newborn infants in the United States are jaundiced; that is, they look yellow. Kernicterus is a form of brain damage caused by excessive jaundice. When babies begin to be affected by excessive jaundice and begin to have brain damage, they become excessively lethargic.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
There are 60,000 miles of blood vessels in every adult human.
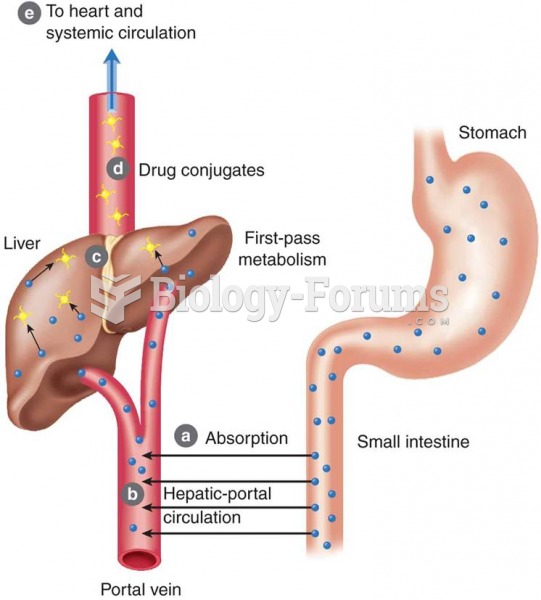 First-pass effect: (a) drugs are absorbed; (b) drugs enter hepatic portal circulation and go directl
First-pass effect: (a) drugs are absorbed; (b) drugs enter hepatic portal circulation and go directl
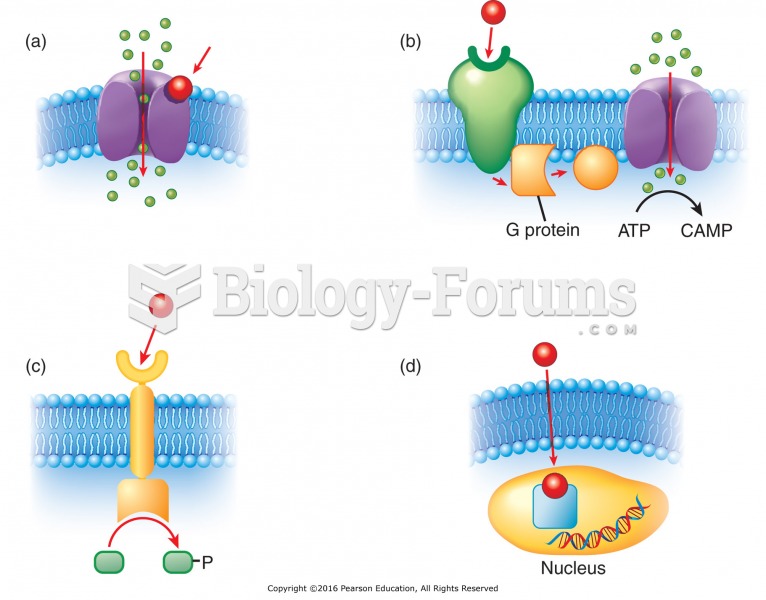 Types of cellular receptors: (a) Drug binds to the receptor opening channel. (b) Drug binds to the ...
Types of cellular receptors: (a) Drug binds to the receptor opening channel. (b) Drug binds to the ...