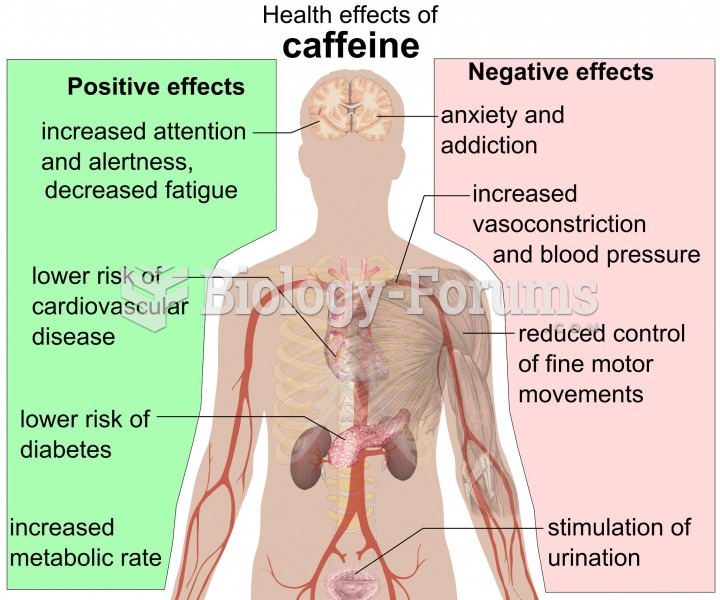
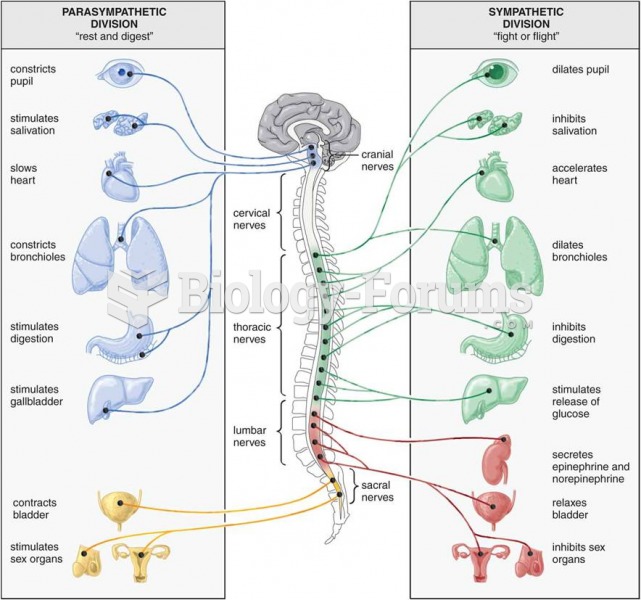
Answer to Question 1
Correct Answer: 4
Rationale 1: The clinical trials end before the drug is released for use by the general public.
Rationale 2: The client's response is not compared with previous clinical trials.
Rationale 3: The efficacy for the drug is not evaluated via the adverse effects.
Rationale 4: Some harmful effects are subtle, take longer to appear, and are not identified until the drug is prescribed to a large number of people; thus, postmarketing surveillance for harmful effects must be reported.
Global Rationale: The nurse reports the adverse effects because some harmful effects are subtle, take longer to appear, and are not identified until the drug is prescribed to a large number of people; thus, postmarketing surveillance for harmful effects must be reported. The clinical trials end before the drug is released for use by the general public. The client's response is not compared with previous clinical trials. The efficacy for the drug is not evaluated via the adverse effects.
Answer to Question 2
Correct Answer: 1,3,4
Rationale 1: Most drugs do not proceed past the preclinical research stage of development because they are found to be either too toxic or just ineffective.
Rationale 2: Client variability and potential drug-to-drug interactions are examined in Phase 3 of the clinical investigation process after Food and Drug Administration (FDA) approval.
Rationale 3: The preclinical stage of development involves extensive testing on human, microbial cells, and animals to determine drug action and to predict whether the drug will cause harm to humans.
Rationale 4: Because lab tests cannot accurately predict human response to a drug, these results are always inconclusive.
Rationale 5: This extensive testing is done by the pharmaceutical company in the preclinical research stage of drug development, not the FDA.
Global Rationale: The true statements include: most drugs do not proceed past the preclinical research stage of development because they are found to be either too toxic or just ineffective; the preclinical stage of developmentinvolves extensive testing on human, microbial cells, and animals to determine drug action and to predict whether the drug will cause harm to humans; and because lab tests cannot accurately predict human response to a drug, these results are always inconclusive. Client variability and potential drug-to-drug interactions are examined in Phase 3 of the clinical investigation process after Food and Drug Administration (FDA) approval. Extensive testing is done by the pharmaceutical company in the preclinical research stage of drug development, not the FDA.