|
|
|
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
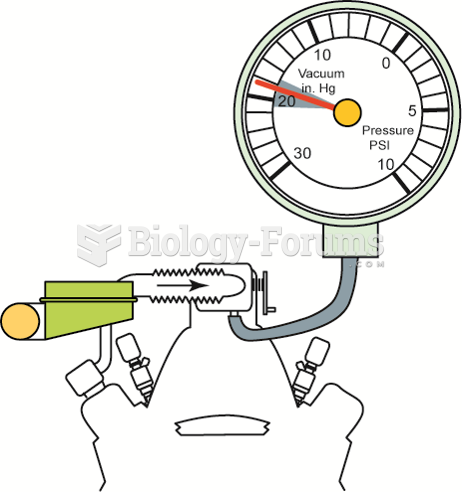 An engine in good mechanical condition should produce 17 to 21 inches Hg of vacuum at idle at sea ...
An engine in good mechanical condition should produce 17 to 21 inches Hg of vacuum at idle at sea ...
 To help locate how far the engine is being rotated, the technician is removing the distributor cap ...
To help locate how far the engine is being rotated, the technician is removing the distributor cap ...